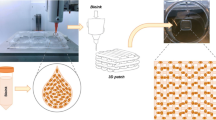
Abstract
Cell-laden cardiac patches have recently been emerging to renew cellular sources for myocardial infarction (MI, commonly know as a heart attack) repair. However, the fabrication of cell-laden patches with porous structure remains challenging due to the limitations of currently available hydrogels and existing processing techniques. The present study utilized a bioprinting technique to fabricate hydrogel patches and characterize them in terms of printability, mechanical and biological properties. Cell-laden hydrogel (or bio-ink) was formulated from alginate dialdehyde (ADA) and gelatin (GEL) to improve the printability, degradability as well as bioactivity. Five groups of hydrogel compositions were designed to investigate the influence of the oxidation degree of ADA and hydrogels concentration on the properties of printed scaffolds. ADA–GEL hydrogels have generally shown favorable for living cells (EA.hy926 cells and hybrid human umbilical vein endothelial cell line). The hydrogel with an oxidation degree of 10% and a concentration ratio of 70/30 (or 10%ADA70–GEL30) demonstrated the best printability among the groups examined. Formulated hydrogels were also bioprinted with the living cells (EA.hy926), and the scaffolds printed were then subject to the cell culture for 7 days. Our results illustrate that the scaffolds bioprinted from 10%ADA70–GEL30 hydrogels had the best homogenous cell distribution and also the highest cell viability. Taken together, in the present study we synthesized a newly formulated bio-ink from ADA and GEL and for the fist time, used them to bioprint cardiac patches, which have the potential to be used in MI repair.











Similar content being viewed by others
References
van Rooij E (2016) Cardiac repair after myocardial infarction. N Engl J Med. https://doi.org/10.1056/nejmcibr1512011
Okamoto T, Akaike T, Sawa T, Miyamoto Y, Van der Vliet A, Maeda H (2001) Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. https://doi.org/10.1074/jbc.M102417200
Fert-Bober J, Leon H, Sawicka J et al (2008) Inhibiting matrix metalloproteinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusion. Basic Res Cardiol. https://doi.org/10.1007/s00395-008-0727-y
Qun Gao C, Sawicki G, Suarez-Pinzon WL et al (2003) Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. https://doi.org/10.1016/S0008-6363(02)00719-8
Doroszko A, Polewicz D, Sawicka J, Richardson JS, Sawicki G, Cheung PY (2009) Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res Cardiol. https://doi.org/10.1007/s00395-009-0035-1
Bozkurt B, Kribbs SB, Clubb FJ et al (1998) Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. https://doi.org/10.1161/01.CIR.97.14.1382
Ye KY, Black LD (2011) Strategies for tissue engineering cardiac constructs to affect functional repair following myocardial infarction. J Cardiovasc Transl Res. https://doi.org/10.1007/s12265-011-9303-1
Segers VFM, Lee RT (2010) Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res. https://doi.org/10.1007/s12265-010-9207-5
Fang R, Tian W, Chen X (2017) Synthesis of injectable alginate hydrogels with muscle-derived stem cells for potential myocardial infarction repair. Appl Sci. https://doi.org/10.3390/app7030252
Bai X, Fang R, Zhang S et al (2013) Self-cross-linkable hydrogels composed of partially oxidized alginate and gelatin for myocardial infarction repair. J Bioact Compat Polym. https://doi.org/10.1177/0883911512473230
Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME (2018) UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. https://doi.org/10.1089/ten.tec.2017.0346
Izadifar M, Babyn P, Kelly ME, Chapman D, Chen X (2017) Bioprinting pattern-dependent electrical/mechanical behavior of cardiac alginate implants: characterization and ex vivo phase-contrast microtomography assessment. Tissue Eng Part C Methods. https://doi.org/10.1089/ten.tec.2017.0222
Shi C, Li Q, Zhao Y et al (2011) Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials 32(10):2508–2515. https://doi.org/10.1016/j.biomaterials.2010.12.026
You F, Eames BF, Chen X (2017) Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering. Int J Mol Sci 18(7):1597. https://doi.org/10.3390/ijms18071597
Chen DXB (2019) Extrusion bioprinting of scaffolds for tissue engineering applications. Springer, Cham
Naghieh S, Sarker M, Izadifar M, Chen X (2018) Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications—a brief review. J Mech Behav Biomed Mater. https://doi.org/10.1016/j.jmbbm.2017.11.037
Ning L, Chen X (2017) A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol J. https://doi.org/10.1002/biot.201600671
Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A (2009) Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120(5):408–416. https://doi.org/10.1161/CIRCULATIONAHA.109.865154
Axpe E, Oyen ML (2016) Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 17(12):1976. https://doi.org/10.3390/ijms17121976
You F, Wu X, Chen X (2017) 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int J Polym Mater Polym Biomater 66(6):299–306. https://doi.org/10.1080/00914037.2016.1201830
You F, Wu X, Zhu N, Lei M, Eames BF, Chen X (2016) 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. In: ACS biomaterials science & engineering, vol 2, pp 1200–1210
Al-Shamkhani A, Duncan R (1995) Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. J Bioact Compat Polym 10(1):4–13. https://doi.org/10.1177/088391159501000102
Balakrishnan B, Lesieur S, Labarre D, Jayakrishnan A (2005) Periodate oxidation of sodium alginate in water and in ethanol–water mixture: a comparative study. Carbohydr Res 340(7):1425–1429. https://doi.org/10.1016/j.carres.2005.02.028
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.Alginate
Chan BP, Leong KW (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17:467–479. https://doi.org/10.1007/s00586-008-0745-3
Rahmany MB, Van Dyke M (2013) Biomimetic approaches to modulate cellular adhesion in biomaterials: a review. Acta Biomater 9(3):5431–5437. https://doi.org/10.1016/j.actbio.2012.11.019
Balakrishnan B, Joshi N, Jayakrishnan A, Banerjee R (2014) Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater 10(8):3650–3663. https://doi.org/10.1016/j.actbio.2014.04.031
Pok S, Myers JD, Madihally SV, Jacot JG (2013) A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater 9(3):5630–5642. https://doi.org/10.1016/j.actbio.2012.10.032
Fang R, Qiao S, Liu Y et al (2015) Sustained co-delivery of BIO and IGF-1 by a novel hybrid hydrogel system to stimulate endogenous cardiac repair in myocardial infarcted rat hearts. Int J Nanomed. https://doi.org/10.2147/IJN.S81451
Sarker B, Papageorgiou DG, Silva R et al (2014) Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J Mater Chem B 2(11):1470. https://doi.org/10.1039/c3tb21509a
Gomez CG, Rinaudo M, Villar MA (2007) Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr Polym 67(3):296–304. https://doi.org/10.1016/j.carbpol.2006.05.025
Zuidema JM, Rivet CJ, Gilbert RJ, Morrison FA (2014) A protocol for rheological characterization of hydrogels for tissue engineering strategies. J Biomed Mater Res Part B Appl Biomater 102(5):1063–1073. https://doi.org/10.1002/jbm.b.33088
Vasconcelos A, Gomes AC, Cavaco-Paulo A (2012) Novel silk fibroin/elastin wound dressings. Acta Biomater 8(8):3049–3060. https://doi.org/10.1016/j.actbio.2012.04.035
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83(2):346–356. https://doi.org/10.1016/0003-2697(77)90043-4
Balakrishnan B, Jayakrishnan A (2005) Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 26(18):3941–3951. https://doi.org/10.1016/j.biomaterials.2004.10.005
Lee HJ, Ahn SH, Kim GH (2012) Three-dimensional collagen/alginate hybrid scaffolds functionalized with a drug delivery system (DDS) for bone tissue regeneration. Chem Mater. 24(5):881–891. https://doi.org/10.1021/cm200733s
Vieira EFS, Cestari AR, Airoldi C, Loh W (2008) Polysaccharide-based hydrogels: preparation, characterization, and drug interaction behaviour. Biomacromol 9(4):1195–1199. https://doi.org/10.1021/bm7011983
Smitha B, Sridhar S, Khan AA (2005) Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur Polym J 41(8):1859–1866. https://doi.org/10.1016/j.eurpolymj.2005.02.018
Kang HA, Shin MS, Yang JW (2002) Preparation and characterization of hydrophobically modified alginate. Polym Bull 47(5):429–435. https://doi.org/10.1007/s002890200005
Wu SC, Chang WH, Dong GC, Chen KY, Chen YS, Yao CH (2011) Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds. J Bioact Compat Polym 26(6):565–577. https://doi.org/10.1177/0883911511423563
Rosellini E, Cristallini C, Barbani N, Vozzi G, Giusti P (2009) Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J Biomed Mater Res Part A 91A(2):447–453. https://doi.org/10.1002/jbm.a.32216
Ye J, Xiong J, Sun R (2012) The fluorescence property of Schiff’s bases of carboxymethyl cellulose. Carbohydr Polym 88(4):1420–1424. https://doi.org/10.1016/j.carbpol.2012.02.030
Acknowledgements
Financial Support from the University of Saskatchewan via scholarship and the Natural Science and Engineering Research Council of Canada via the Discovery Grant is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
You, F., Wu, X., Kelly, M. et al. Bioprinting and in vitro characterization of alginate dialdehyde–gelatin hydrogel bio-ink. Bio-des. Manuf. 3, 48–59 (2020). https://doi.org/10.1007/s42242-020-00058-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-020-00058-8