Abstract
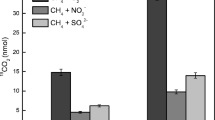
Anaerobic methane oxidation is a key process for methane reduction and nitrogen removal in eutrophic lakes. It is very important to know the distribution patterns of anaerobic methane oxidation activity and the related microbes in the typical plateau eutrophic lakes. Here, we aimed to characterize the anaerobic methane oxidation activity and to reveal the correlations to biological/non-biological factors along eutrophic gradients in Dianchi Lake. The anaerobic methane oxidation activity was analyzed by anaerobic incubation and gas chromatography. Candidatus Methylomirabilis was analyzed by Illumina Miseq 16S ribosomal RNA gene sequencing. The methane oxidation activities ranged from 1.71 to 5.07 μg/g day−1 in Dianchi Lake. These activities differed significantly among the four zones (p = 0.000), with more activity in the high eutrophication zone (Caohai). The relative abundance of Candidatus Methylomirabilis ranged from 84.86 to 98.13%, with no significant changes evident in the four research zones in Dianchi Lake. The anaerobic methane oxidation activity was significantly positively related to TN, NO3−-N, TC, and Candidatus Methylomirabilis abundance, and significantly negatively related to TP and pH. Thus, our study showed anaerobic methane oxidation occurred in Dianchi sediment and the activity of anaerobic methane oxidation was associated with the level of eutrophication.



Similar content being viewed by others
References
Bastviken, D., Cole, J., Pace, M., & Tranvik, L. (2004). Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochemical Cycles, 18(4), 305–313.
Boetius, A., Ravenschlag, K., Schubert, C. J., Rickert, D., Widdel, F., Gieseke, A., et al. (2000). A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature, 407(6804), 623.
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5), 335–336.
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berglyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME Journal Multidisciplinary Journal of Microbial Ecology, 6(8), 1621–1624.
Chen, Y., Bai, X., Liu, W., Nanjing, B., & China, P. R. (2007). A primary study of the methane flux on the water-air interface of eight lakes in winter, China. Journal of Lake Sciences, 19(1), 11–17.
Ding, J., Fu, L., Ding, Z.-W., Lu, Y.-Z., Cheng, S. H., & Zeng, R. J. (2016). Environmental evaluation of coexistence of denitrifying anaerobic methane-oxidizing archaea and bacteria in a paddy field. Applied Microbiology and Biotechnology, 100(1), 439–446.
Ettwig, K. F., van Alen, T., van de Pas-Schoonen, K. T., Jetten, M. S. M., & Strous, M. (2009). Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Applied and Environmental Microbiology, 75(11), 3656–3662.
He, Z., Zhang, Q., Feng, Y., Luo, H., Pan, X., & Gadd, G. M. (2017). Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Science of the Total Environment, 610-611, 759.
Hoehler, T. M., Alperin, M. J., Albert, D. B., & Martens, C. S. (1994). Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium. Global Biogeochemical Cycles, 8(4), 451–463.
Kirschke, S., Bousquet, P., Ciais, P., Saunois, M., Canadell, J. G., Dlugokencky, E. J., et al. (2013). Three decades of global methane sources and sinks. Nature Geoscience, 6(10), 813–823.
Le, C., Zha, Y., Li, Y., Sun, D., Lu, H., & Yin, B. (2010). Eutrophication of lake waters in China: cost, causes, and control. Environmental Management, 45(4), 662. https://doi.org/10.1007/s00267-010-9440-3.
Luesken, F. A., van Alen, T. A., van der Biezen, E., Frijters, C., Toonen, G., Kampman, C., et al. (2011). Diversity and enrichment of nitrite-dependent anaerobic methane oxidizing bacteria from wastewater sludge. Applied Microbiology and Biotechnology, 92(4), 845–854.
Naqvi, S. W. A., Lam, P., Narvenkar, G., Sarkar, A., Naik, H., Pratihary, A., et al. (2018). Methane stimulates massive nitrogen loss from freshwater reservoirs in India. Nature Communications, 9. https://doi.org/10.1038/s41467-018-03607-z.
Podgrajsek, E., Sahlée, E., Bastviken, D., Natchimuthu, S., Kljun, N., Chmiel, H. E., et al. (2016). Methane fluxes from a small boreal lake measured with the eddy covariance method. Limnology & Oceanography, 61(S1). https://doi.org/10.1002/lno.10245.
Qian, Y. T., Xiao-Mei, Y. E., Chang, Z. Z., Jing, D. U., & Pan, J. C. (2011). Methane production characteristics of water hyacinth from different water areas. China Environmental Science, 31(9), 1509–1515.
Raghoebarsing, A. A., Pol, A., van de Pas-Schoonen, K. T., Smolders, A. J. P., Ettwig, K. F., Rijpstra, W. I. C., et al. (2006). A microbial consortium couples anaerobic methane oxidation to denitrification. Nature, 440(7086), 918. https://doi.org/10.1038/nature04617.
Reeburgh, W. S. (2010). Oceanic methane biogeochemistry. Chemical Reviews, 38(20). https://doi.org/10.1021/cr050362v.
Segarra, K. E., Schubotz, F., Samarkin, V., Yoshinaga, M. Y., Hinrichs, K. U., & Joye, S. B. (2015). High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nature Communications, 6, 7477. https://doi.org/10.1038/ncomms8477.
Shen, L.-D., Wu, H.-S., Liu, X., & Li, J. (2017). Cooccurrence and potential role of nitrite- and nitrate-dependent methanotrophs in freshwater marsh sediments. Water Research, 123, 162–172.
Tanja, M., & Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 27(21), 2957–2963.
van Kessel, M. A. H. J., Stultiens, K., Slegers, M. F. W., Cruz, S. G., Jetten, M. S. M., Kartal, B., et al. (2018). Current perspectives on the application of N-damo and anammox in wastewater treatment. Current Opinion in Biotechnology, 50, 222–227.
Wang, H., Lu, J., Wang, W., Yang, L., & Yin, C. (2006). Methane fluxes from the littoral zone of hypereutrophic Taihu Lake, China. Journal of Geophysical Research Atmospheres, 111, 4093–4100.
Wang, W., Zeng, C., Sardans, J., Wang, C., Tong, C., & Peñuelas, J. (2018). Soil methane production, anaerobic and aerobic oxidation in porewater of wetland soils of the Minjiang River Estuarine, China. Wetlands, 1–14.
Wassmann, R., Neue, H. U., Bueno, C., Lantin, R. S., Alberto, M. C. R., Buendia, L. V., et al. (1998). Methane production capacities of different rice soils derived from inherent and exogenous substrates. Plant & Soil, 203(2), 227–237.
Welte, C. U., Rasigraf, O., Vaksmaa, A., Versantvoort, W., Arshad, A., Op den Camp, H. J. M., et al. (2016). Nitrate- and nitrite-dependent anaerobic oxidation of methane. Environmental Microbiology Reports, 8(6), 941–955.
Yang, Y., Zhao, Q., Cui, Y., Wang, Y., Xie, S., & Liu, Y. (2016). Spatio-temporal variation of sediment methanotrophic microorganisms in a large eutrophic Lake. Microbial Ecology, 71(1), 9–17.
Yang, Y., Li, N., Wei, W., Li, B., Xie, S., & Yong, L. (2017). Vertical profiles of sediment methanogenic potential and communities in two plateau freshwater lakes. Biogeosciences, 14(2), 1–38.
Yang, Y., Chen, J., Li, B., Liu, Y., & Xie, S. (2018). Anaerobic methane oxidation potential and bacteria in freshwater lakes: Seasonal changes and the influence of trophic status. Systematic and Applied Microbiology, 41(6), 650–657.
Funding
This study was financially supported by the National Natural Science Foundation of China (31700411 and 41663008), the Programs of Science and Technology Department Foundation of Yunnan Province (2018FD007, 2016FD014, and 2018 BC001), the Undergraduate Training Programs for Innovation and Entrepreneurship of Yunnan Province (201710673047), and the Yunnan Key Laboratory for Plateau Mountain Ecology and Restoration of Degraded Environments (2018DG005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Chen, Y., Luo, M. et al. Comparison of Anaerobic Methane Oxidation in Different Sediment Habitats of Dianchi Lake. Water Air Soil Pollut 231, 491 (2020). https://doi.org/10.1007/s11270-020-04868-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04868-5