Abstract
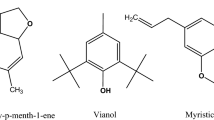
We investigated experimentally the composition of volatile isolates from the leaves and seeds of fraxinus angustifolia Vahl and their antimicrobial activity. The experimental work was supported by a theoretical study, using the Functional Density Theory. The calculations were performed at the B3LYP/6–31 g-d, p), in order to determine the structures of major volatile compounds and the different descriptors of reactivity and biological activity. The chemical composition of the volatile isolates obtained by hydrodistillation of the leaves and seeds are evaluated by the analytical method of gas chromatography-mass spectrometry (GC-MS). We found that the leaves are composed of abundant compounds, specifically, docosane methyl (30.2%), n-Pentacosane (28.5), α-cadinol (9.0%) and T-muurolol (5.9%). Moreover, the isolate from the seeds, is found to be mainly composed of α-cadinol (23.2%) and epi-methyljasmonate (34. 2%). Additionally, we perform antimicrobial activity tests on the volatile isolated using the zone of inhibition (agar disk-diffusion method) of four bacteria strains, mainly, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli. The values of leaves range from 100 to 300 µg of volatile compounds, whereas for the seeds the values lie between 20 and 300 µg. Furthermore, antifungal susceptibility tests are conducted on two yeast strains: Saccharomyces cerevisiae and Candida albicans. The seeds have a better inhibition than the leaves, with 20 and 300 µg respectively. The chemical compositions of the volatile fractions of leaves and seeds are correlated with the antimicrobial results. The reactivity descriptors are calculated to determine stability and microbiological activity of the above compounds. The Isopimaradiene presents the higher microbiological activity and the most stable T-muurololis.
Graphic Abstract








Similar content being viewed by others
References
Boucher, H.W., Talbot, G.H., Bradley, J.S., et al.: Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases Society of America. Clin. Infect Dis. 48, 1–12 (2009). https://doi.org/10.1086/595011
Giamarellou, H.: Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int. J. Antimicrob. Agents 36, S50–S54 (2010)
Coates, A., Hu, Y., Bax, R., Page, C.: The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 1, pages895-910 (2002)
Marasini, B.P., Baral, P., Aryal, P., et al.: Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Biomed. Res. Int. (2015). https://doi.org/10.1155/2015/265425
Bobbarala, V.: (2012) Edited by Varaprasad Bobbarala
Manandhar, S., Luitel, S., Dahal, R.K.: In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. (2019). https://doi.org/10.1155/2019/1895340
Organisation Mondiale de la Santé: (2020) Thèmes de santé Médecine traditionnelle. https://www.who.int/topics/traditional_medicine/fr/
Medina, A.L., Lucero, M.E., Holguin, F.O., et al.: Composition and antimicrobial activity of Anemopsis californica leaf oil. J. Agric. Food Chem. 53, 8694–8698 (2005). https://doi.org/10.1021/jf0511244
Al-Haj, N., Reem, A., Al-Shamahy, H., et al.: Antimicrobial activity of five yemeni medicinal plants against selected human pathogenic bacteria and fungi. Am. J. Plant Sci. 10, 1699–1707 (2019). https://doi.org/10.4236/ajps.2019.1010121
Caccioni, D.R.L., Guizzardi, M.: Inhibition of germination and growth of fruit and vegetable postharvest pathogenic fungi by essential oil components. J. Essent. Oil Res. 6, 173–179 (1994). https://doi.org/10.1080/10412905.1994.9698349
Cimanga, K., Apers, S., De Bruyne, T., et al.: Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 79, 213–220 (2002). https://doi.org/10.1080/10412905.2002.9699894
Bozin, B., Mimica-Dukic, N., Simin, N., Anackov, G.: Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 54, 1822–1828 (2006). https://doi.org/10.1021/jf051922u
Cordery, A., Rao, A.P., Ravishankar, S.: Antimicrobial activities of essential oils, plant extracts and their applications in foods—a review. J. Agric. Environ. Sci. 7, 76–89 (2018). https://doi.org/10.15640/jaes.v7n2a9
Preedy, V.: Essential Oils in Food Preservation, Flavor and Safety. Academic Press, Cambridge (2015)
Youdim, K.A., Deans, S.G., Finlayson, H.J.: The antioxidant properties of thyme (Thymus zygis L.) essential oil: an inhibitor of lipid peroxidation and a free radical scavenger. J. Essent. Oil Res. 14, 210–215 (2002). https://doi.org/10.1080/10412905.2002.9699825
Fasseas, M.K., Mountzouris, K.C., Tarantilis, P.A., et al.: Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 106, 1188–1194 (2008). https://doi.org/10.1016/j.foodchem.2007.07.060
Turek, C., Stintzing, F.C.: Stability of essential oils: a review. Compr. Rev. Food Sci. Food Saf. 12, 40–53 (2013). https://doi.org/10.1111/1541-4337.12006
Sarfraz, I., Rasul, A., Jabeen, F., et al.: Fraxinus: a plant with versatile pharmacological and biological activities. Evid. -Based Complement Altern. Med. (2017). https://doi.org/10.1155/2017/4269868
Atmani, D., Chaher, N., Berboucha, M., et al.: Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 112, 303–309 (2009). https://doi.org/10.1016/j.foodchem.2008.05.077
Berboucha, M., Ayouni, K., Atmani, D., et al.: Kinetic study on the inhibition of xanthine oxidase by extracts from two selected algerian plants traditionally used for the treatment of inflammatory diseases. J. Med. Food 13, 896–904 (2010)
Ayouni, K., Berboucha-rahmani, M., Kim, H.K., et al.: Angustifolia leaf and stem bark extracts. Ind. Crop Prod. (2016). https://doi.org/10.1016/j.indcrop.2016.01.001
European Medicines Agency: (2012) No Title. Comm Herb Med Prod EMA/HMPC/239271/2011, 27 March 2012
Heatley, N.G.: A rapid method for the assay of penicillin. Biochem. J. 38, 61–65 (1944). https://doi.org/10.1139/cjr47e-002
Hudzicki, J.: Kirby-Bauer disk diffusion susceptibility test protocol author information. Am Soc Microbiol 1–13 (2009)
Van Den Doo, H., Kratz, D.P.: A generalisation of the retention index system including linear temperature programme Gas-Liquid partition chromatography. J. Chromatogr. 11, 463–471 (1963). https://doi.org/10.1007/978-3-319-70262-9_7
Wiley, J.: Wiley Registry of Mass Spectral Data, with NIST 2008, 9th edn. Wiley, Hoboken (2008)
Adams, R.P.: Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th edn. Allured publishing corporation, Carol Stream (2007)
Joulain, D., König, W.A.: The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. EB-Verlag, Berlin (1998)
Cavaleiro, C., Salgueiro, L.R., Miguel, M.G., Da ProençaCunha, A.: Analysis by gas chromatography-mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A 1033, 187–190 (2004). https://doi.org/10.1016/j.chroma.2004.01.005
Cavaleiro, C., Gonçalves, M.J., Serra, D., et al.: Composition of a volatile extract of Eryngium duriaei subsp. juresianum (M. Laínz) M. Laínz, signalised by the antifungal activity. J. Pharm. Biomed. Anal. 54, 619–622 (2011). https://doi.org/10.1016/j.jpba.2010.09.039
Hammer, K.A., Carson, C.F., Riley, T.V.: Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86, 985–990 (1999). https://doi.org/10.1046/j.1365-2672.1999.00780.x
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Ja, R.: Gaussian 03, Revision B.01. Gaussian Inc., Wallingford (2003)
Farooq, U., Ayub, K., Hashmi, M.A., et al.: A new rosane-type diterpenoid from Stachys parviflora and its density functional theory studies. Nat. Prod. Res. 29, 813–819 (2014). https://doi.org/10.1080/14786419.2014.987775
Liu, J., Lv, B., Liu, H., et al.: Insight into the C-F bond mechanism of molecular analogs for antibacterial drug design. Nat. Prod. Res. 32, 1312–1315 (2017). https://doi.org/10.1080/14786419.2017.1340290
Lee, C., Yang, W., Parr, R.G.: Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. B 37, 785 (1988)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 82, 284 (1985)
McLean, A.D., Chandler, G.S.: Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 72, 5639–5648 (1980). https://doi.org/10.1063/1.438980
Parr, R.G., Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983)
Parr, R.G., Donnelly, R.A., Levy, M., Palke, W.E.: Electronegativity: the density functional viewpoint. J. Chem. Phys. 68, 3801–3807 (1978). https://doi.org/10.1063/1.436185
Koopmans, T.: Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1, 104–113 (1934). https://doi.org/10.1016/S0031-8914(34)90011-2
Kostova, I., Iossifova, T.: Chemical components of Fraxinus species. Fitoterapia 78, 85–106 (2007). https://doi.org/10.1016/j.fitote.2006.08.002
Hosny, M., Çaliş, İ, Nishibe, S.: Secoiridoids from Fraxinus angustifolia. Planta Med. 57, 1991 (1991)
Markovic, I., Norris, D.M., Phillips, J.K., Webster, F.X.: Volatiles involved in the nonhost rejection of Fraxinus pennsylvanica by Lymantria dispar larvae. J. Agric. Food Chem. 44, 929–935 (1996). https://doi.org/10.1021/jf9502111
M’sou, S., Alifriqui, M., Romane, A.: Phytochemical study and biological effects of the essential oil of Fraxinus dimorpha Coss & Durieu§. Nat. Prod. Res. 31, 2797–2800 (2017). https://doi.org/10.1080/14786419.2017.1294173
Giardinieri, A., Schicchi, R., Geraci, A., et al.: Fixed oil from seeds of narrow-leaved ash (F. angustifolia subsp. angustifolia): chemical profile, antioxidant and antiproliferative activities. Food Res. Int. 119, 369–377 (2019). https://doi.org/10.1016/j.foodres.2019.02.013
Wasternack, C., Hause, B.: Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058 (2013). https://doi.org/10.1093/aob/mct067
Wang, J., Wu, D., Wang, Y., Xie, D.: Jasmonate action in plant defense against insects Jiaojiao. J. Exp. Biol. 70, 3391–3400 (2019). https://doi.org/10.1093/jxb/erz174
Hmamouchi, M., Hamamouchi, J., Zouhdi, M., Bessiere, J.M.: Chemical and antimicrobial properties of essential oils of five Moroccan pinaceae. J. Essent. Oil Res. 13, 298–302 (2001). https://doi.org/10.1080/10412905.2001.9699699
Astani, A., Reichling, J., Schnitzler, P.: Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phyther. Res. 24, 673–679 (2009). https://doi.org/10.1002/ptr2955
Martin, S., Padilla, E., Ocete, M.A., et al.: Anti-inflammatory activity of the essential oil of Bupleurum fruticescens. Planta Med. 59, 533–536 (1993). https://doi.org/10.1055/s-2006-959755
Mabou, F.D., Ngnokam, D., Harakat, D., Voutquenne-Nazabadioko, L.: New oleanane-type saponins: leptocarposide B-D, from Ludwigia leptocarpa (Onagraceae). Phytochem. Lett. 14, 159–164 (2015)
Loizzo, M.R., Tundis, R., Menichini, F., et al.: Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell. Prolif. 41, 1002–1012 (2008). https://doi.org/10.1111/j.1365-2184.2008.00561.x
Su, Y.C., Hsu, K.P., Wang, E.I.C., Ho, C.L.: Composition, anticancer, and antimicrobial activities in vitro of the heartwood essential oil of Cunninghamia lanceolata var. konishii from Taiwan. Nat. Prod. Commun. 7, 1245–1247 (2012). https://doi.org/10.1177/1934578x1200700938
Tabanca, N., Demirci, F., et al.: Composition and antimicrobial activity of the essential oil of Origanum dolichosiphon P. H. Davis. Chem. Nat. Compd. 37, 238–241 (2001)
Guy, I., Charles, B., Guinaudeau, H., et al.: Essential oils from leaves of two Paraguayan Rutaceae: Zanthoxylum hyemale A. St. Hil. And Z. Naranjillo Griseb. J. Essent. Oil Res. 13, 200–201 (2001). https://doi.org/10.1080/10412905.2001.9699663
Liu, X., Cai, J., Chen, H., et al.: Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 141, 103980 (2020). https://doi.org/10.1016/j.micpath.2020.103980
Kotan, R., Kordali, S., Cakir, A.: Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Fur Naturforsch Sect. C J. Biosci. 62, 507–513 (2007). https://doi.org/10.1515/znc-2007-7-808
Cheng, S.S., Chung, M.J., Lin, C.Y., et al.: Phytochemicals from Cunninghamia konishii Hayata act as antifungal agents. J. Agric. Food Chem. 60, 124–128 (2012). https://doi.org/10.1021/jf2042196
Frankerankenbach, G.M., Hollingshead, J.A., Horenziak, S.A.: (2017) Substrates comprising malodor reduction compositions. United States Pat Appl
Porto, T.S., Rangel, R., Furtado, N.A.J.C., De Carvalho, T.C., Martins, C.H.G., Veneziani, R.C.S., DaCosta, F.B., Vinholis, A.H.C., Cunha, W.R., Heleno, V.C.G., Ambrosio, S.R.: Pimarane-type diterpenes: antimicrobial activity against oral pathogens. Molecules 14(1), 191–199 (2009). https://doi.org/10.3390/molecules14010191
Ambrósio, S.R., Arakawa, N.S., Esperandim, V.R., et al.: Trypanocidal activity of pimarane diterpenes from Viguiera arenaria (Asteraceae). Phyther. Res. 22, 1413–1415 (2008)
Pongprayoon, U., Sematong, T., Tuchinda, P., et al.: Topical antiinflammatory activity of two pimarane diterpenes from Kaempferia pulchra. Phyther. Res. 10, 534–535 (1996)
Mansour, A.M.: Coordination behavior of sulfamethazine drug towards Ru(III) and Pt(II) ions: synthesis, spectral, DFT, magnetic, electrochemical and biological activity studies. Inorg. Chim. Acta 394, 436–445 (2013). https://doi.org/10.1016/j.ica.2012.08.025
Tidjani-rahmouni, N., Bensiradj, H., Djebbar, S., Benali-baitich, O.: Synthesis, characterization, electrochemical studies and DFT calculations of amino acids ternary complexes of copper (II) with isonitrosoacetophenone. Biological activities. J. Mol. Struct. 1075, 254–263 (2014). https://doi.org/10.1016/j.molstruc.2014.06.067
Zhang, F., Tang, Y., Cao, Z., et al.: Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid. Corros. Sci. 61, 1–9 (2012). https://doi.org/10.1016/j.corsci.2012.03.045
Andrade-Ochoa, S., Nevárez-Moorillón, G.V., Sánchez-Torres, L.E., et al.: Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement Altern. Med. 15, 1–11 (2015). https://doi.org/10.1186/s12906-015-0858-2
Frau, J., Glossman-Mitnik, D.: Conceptual DFT study of the local chemical reactivity of the colored BISARG melanoidin and its protonated derivative. Front. Chem. 6, 1–9 (2018). https://doi.org/10.3389/fchem.2018.00136
Koroch, A.R., Juliani, H.R.: Bioactivity of essential oils and their components. In: Falvours and Fragrances Chemistry, pp. 87–115. Springer, Berlin (2007)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zouaghi, N., Houda Bensiradj, N.E., Cavaleiro, C. et al. Phytochemical Study and Antibacterial Effects of Fraxinus angustifolia Vahl (Algeria): Experimental and Computational Investigations. Waste Biomass Valor 12, 3605–3616 (2021). https://doi.org/10.1007/s12649-020-01240-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01240-w