Abstract
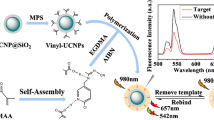
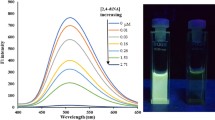
A fluorometric method based on molecularly imprinted upconversion fluorescence test strip was developed for the determination of tyramine. It exploited the green fluorescence of upconversion nanoparticles (UCNPs) and the specific recognition property of molecularly imprinted polymers (MIPs). UCNPs were attached to filter paper with glue, and MIPs were prepared via in situ polymerization on the surface of UCNPs by using tyramine as template, methacrylic acid as functional monomer, and ethylene glycol dimethacrylate as cross-linker. The green fluorescence of the test strip, with excitation/emission wavelength 980/550 nm, was enhanced by tyramine. The test strip was suitable for the determination of tyramine in the linear range 1.0–100.0 mg L−1, and a relatively low limit of detection (0.2 mg L−1) was achieved. The test strip also worked well for the quantitation of tyramine in spiked red wine and mature vinegar. Recoveries are ranged from 84.9 to 99.9%. The relative standard deviations are below 5.6%.

Graphical abstract




Similar content being viewed by others
References
Ten Brink B, Damink C, Joosten HM, Huis in 't Veld JH (1990) Occurrence and formation of biologically active amines in foods. Int J Food Microbiol 11(1):73–84. https://doi.org/10.1016/0168-1605(90)90040-c
Alizadeh N, Kamalabadi M, Mohammadi A (2017) Determination of histamine and tyramine in canned fish samples by headspace solid-phase microextraction based on a nanostructured polypyrrole fiber followed by ion mobility spectrometry. Food Anal Methods 10(9):3001–3008. https://doi.org/10.1007/s12161-017-0860-z
Sheng W, Sun CC, Fang GZ, Wu XN, Hu GS, Zhang Y, Wang S (2016) Development of an enzyme-linked immunosorbent assay for the detection of tyramine as an index of freshness in meat and seafood. J Agric Food Chem 64(46):8944–8949. https://doi.org/10.1021/acs.jafc.6b04422
Costantini A, Vaudano E, Del Prete V, Danei M, Garcia-Moruno E (2009) Biogenic amine production by contaminating bacteria found in starter preparations used in winemaking. J Agric Food Chem 57(22):10664–10669. https://doi.org/10.1021/jf9025426
Beneduce L, Romano A, Capozzi V, Lucas P, Barnavon L, Bach B, Vuchot P, Grieco F, Spano G (2010) Biogenic amine in wines. Ann Microbiol 60(4):573–578. https://doi.org/10.1007/s13213-010-0094-4
Ke RH, Wei ZS, Bogdal C, Goktas RK, Xiao RY (2018) Profiling wines in China for the biogenic amines: a nationwide survey and pharmacokinetic fate modelling. Food Chem 250:268–275. https://doi.org/10.1016/j.foodchem.2018.01.040
Dong H, Xiao KJ (2017) Modified QuEChERS combined with ultrahigh performance liquid chromatography tandem mass spectrometry to determine seven biogenic amines in Chinese traditional condiment soy sauce. Food Chem 229:502–508. https://doi.org/10.1016/j.foodchem.2017.02.120
Khan MZH, Liu XQ, Zhu J, Ma F, Hu W, Liu XH (2018) Electrochemical detection of tyramine with ITO/APTES/ErGO electrode and its application in real sample analysis. Biosens Bioelectron 108:76–81. https://doi.org/10.10164/j.bios.2018.02.042
Kaur N, Kaur M, Chopra S, Singh J, Kuwar A, Singh N (2018) Fe (III) conjugated fluorescent organic nanoparticles for ratiometric detection of tyramine in aqueous medium: a novel method to determine food quality. Food Chem 245:1257–1261. https://doi.org/10.1016/j.foodchem.2017.11.097
Zhang DW, Liu HL, Geng WT, Wang YP (2019) A dual-function molecularly imprinted optopolymer based on quantum dots-grafted covalent-organic frameworks for the sensitive detection of tyramine in fermented meat products. Food Chem 277:639–645. https://doi.org/10.1016/j.foodchem.2018.10.147
Jang H, Park J-H, Oh J, Kim K, Kim M-G (2019) Advanced colorimetric paper sensors using color focusing effect based on asymmetric flow of fluid. Acs Sensors 4(4):1103–1108. https://doi.org/10.1021/acssensors.9b00390
Colozza N, Kehe K, Dionisi G, Popp T, Tsoutsoulopoulos A, Steinritz D, Moscone D, Arduini F (2019) A wearable origami-like paper-based electrochemical biosensor for sulfur mustard detection. Biosens Bioelectron 129:15–23. https://doi.org/10.1016/j.bios.2019.01.002
Fernandes SC, Walz JA, Wilson DJ, Brooks JC, Mace CR (2017) Beyond wicking: expanding the role of patterned paper as the foundation for an analytical platform. Anal Chem 89(11):5655–5665. https://doi.org/10.1021/acs.analchem.6b03860
Liu LY, Yang DT, Liu GZ (2019) Signal amplification strategies for paper-based analytical devices. Biosens Bioelectron 136:60–75. https://doi.org/10.1016/j.bios.2019.04.043
Wang SM, Ge L, Li L, Yan M, Ge SG, Yu JH (2013) Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosens Bioelectron 50:262–268. https://doi.org/10.1016/j.bios.2013.07.003
Liu W, Guo YM, Luo J, Kou J, Zheng HY, Li BX, Zhang ZJ (2015) A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim Acta A 141:51–57. https://doi.org/10.1016/j.saa.2015.01.020
Sun XL, Jian YN, Wang H, Ge SG, Yan M, Yu JH (2019) Ultrasensitive microfluidic paper-based electrochemical biosensor based on molecularly imprinted film and boronate affinity sandwich assay for glycoprotein detection. ACS Appl Mater Interfaces 11(17):16198–16206. https://doi.org/10.1021/acsami.9b02005
Zhang C, Cui HY, Han YF, Yu FF, Shi XM (2018) Development of a biomimetic enzyme-linked immunosorbent assay based on molecularly imprinted polymers on paper for the detection of carbaryl. Food Chem 240:893–897. https://doi.org/10.1016/j.foodchem.2017.07.109
Xiao L, Zhang Z, Wu CC, Han LY, Zhang HY (2017) Molecularly imprinted polymer grafted paper-based method for the detection of 17β-estradiol. Food Chem 221:82–86. https://doi.org/10.1016/j.foodchem.2016.10.062
Sari E, Uzek R, Merkoci A (2019) Paper based photoluminescent sensing platform with recognition sites for tributyltin. ACS Sensors 4(3):645–653. https://doi.org/10.1021/acssensors.8b01396
Fang M, Zhou L, Zhang H, Liu L, Gong Z-Y (2019) A molecularly imprinted polymers/carbon dots-grafted paper sensor for 3-monochloropropane-1,2-diol determination. Food Chem 274:156–161. https://doi.org/10.1016/j.foodchem.2018.08.133
Wang JX, Dai JD, Xu YQ, Dai XH, Zhang YL, Shi WD, Sellergren B, Pan GQ (2019) Molecularly imprinted fluorescent test strip for direct, rapid, and visual dopamine detection in tiny amount of biofluid. Small 15(1):e1803913. https://doi.org/10.1002/smll.201803913
Wang W, Li HJ, Yin MY, Wang KW, Deng QL, Wang S, Zhang YK (2018) Highly selective and sensitive sensing of 2,4,6-trinitrophenol in beverages based on guanidine functionalized upconversion fluorescent nanoparticles. Sensor Actuat B-Chem 255:1422–1429. https://doi.org/10.1016/j.snb.2017.08.143
Yin MY, Wu C, Li HJ, Jia ZX, Deng QL, Wang S, Zhang CY (2019) Simultaneous sensing of seven pathogenic bacteria by guanidine-functionalized upconversion fluorescent nanoparticles. ACS Omega 4(5):8953–8959. https://doi.org/10.1021/acsomega.9b00775
China National Standardizing Committee (2016) Method for determination of tyramine in wine and condiments dairy products HPLC method, GB5009.208-2016-kw
Safdarian M, Ramezani Z, Ghadiri AA (2016) Facile synthesis of magnetic molecularly imprinted polymer: perphenazine template and its application in urine and plasma analysis. J Chromatogr A 1455:28–36. https://doi.org/10.1016/j.chroma.2016.05.083
Safdarian M, Ramezani Z (2019) Rapid microwave-assisted distillation-precipitation polymerization for the synthesis of magnetic molecular imprinted polymers coupled to HPTLC determination of perphenazine in human urine. New J Chem 43(1):48–57. https://doi.org/10.1039/c8nj05062g
Hao TF, Wei X, Nie YJ, Xu YQ, Yan YS, Zhou ZP (2016) An eco-friendly molecularly imprinted fluorescence composite material based on carbon dots for fluorescent detection of 4-nitrophenol. Microchim Acta 183(7):2197–2203. https://doi.org/10.1007/s00604-016-1851-2
Zhang C, Cui HY, Cai JR, Duan YQ, Liu Y (2015) Development of fluorescence sensing material based on CdSe/ZnS quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and Chinese cabbage. J Agric Food Chem 63(20):4966–4972. https://doi.org/10.1021/acs.jafc.5b01072
Jiao Z, Li JW, Mo LJ, Liang JM, Fan HB (2018) A molecularly imprinted chitosan doped with carbon quantum dots for fluorometric determination of perfluorooctane sulfonate. Microchim Acta 185(10):9. https://doi.org/10.1007/s00604-018-2996-y
Wang QH, Zhang DD (2018) A novel fluorescence sensing method based on quantum dot-graphene and a molecular imprinting technique for the detection of tyramine in rice wine. Anal Methods 10(31):3884–3889. https://doi.org/10.1039/c8ay01117f
Wang HH, Lu YY, Wang L, Chen HQ (2019) Detection of tyramine and tyrosinase activity using red region emission NaGdF4:Yb,Er@NaYF4 upconversion nanoparticles. Talanta 197:558–566. https://doi.org/10.1016/j.talanta.2019.01.079
Funding
The authors received financial support provided by the Ministry of Science and Technology of China (Project No.2016YFD0401101) and the National Natural Science Foundation of China (Project No.21375094).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4411 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Fan, F., Fang, G. et al. Fluorometric determination of tyramine by molecularly imprinted upconversion fluorescence test strip. Microchim Acta 187, 573 (2020). https://doi.org/10.1007/s00604-020-04554-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04554-7