Abstract
Sn2+ ions were introduced in Cs-Pb-Br nanocrystals (NCs) to test the change of crystal structure and photoluminescence (PL) property. In the cases of Sn/Pb ratios less than 20%, the CsPb1-xSnxBr3 NCs revealed CsPbBr3 crystal structure. With increasing the Sn/Pb ratio, Cs-Pb-Sn-Br NCs exhibited a mixture phase of CsPbBr3 and Cs4PbBr6. The NCs show bright PLQYs (87%) for Sn/Pb molar ratio of 30%. This is ascribed to little amount Cs4PbBr6 decreased the electrons and holes non-radiation recombination of CsPbBr3. With increasing the Sn/Pb ratio to 80%, the Cs4Pb1-xSnxBr6 NCs exhibited pure Cs4PbBr6 phase structure. Furthermore, a weak blue-emitting peak around 450 nm was observed for the Cs4Pb1-xSnxBr6 NCs. This confirms that Sn2+ ions were successfully introduced into Cs4PbBr6 structure. The morphologies of samples changed from initial cubic to hexagonal with increasing Sn/Pb ratios from 20 to 80%. The excitons and free carrier dynamics of the NCs were discussed.
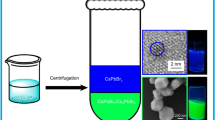
Graphical abstract








Similar content being viewed by others
References
Cha JH, Han JH, Yin W, Park C, Park Y, Ahn TK, Cho JH, Jung DY (2017) Photoresponse of CsPbBr3 and Cs4PbBr6 perovskite single crystals. J Phys Chem Lett 8:565–570
Chen D, Wan Z, Chen X, Yuan Y, Zhong J (2016) Large scale room temperature synthesis and optical properties of perovskite related Cs4PbBr6 fluorophores. J Mater Chem C 4:10646–10653
Chen X, Chen D, Li J, Fang G, Sheng H, Zhong J (2018a) Tunable CsPbBr3/Cs4PbBr6 phase transformation and their optical spectroscopic properties. Dalton Trans 47:5670–5678
Chen X, Zhang F, Ge Y, Shi L, Huang S, Tang J, Zhang L, Zou B, Zhong H (2018b) Centimeter sized Cs4PbBr6 crystals with embedded CsPbBr3 nanocrystals showing superior PL: nonstoichiometry induced transformation and light-emitting applications. Adv Funct Mater 28:1706567
Ding Y, Li T, Li X, Tsiwah EA, Liu C, Gao P, Zeng T, Chen Y, Zhao X, Xie Y (2019) Tin-assisted growth of all-inorganic perovskite nanoplatelets with controllable morphologies and complementary emissions. CrystEngComm 21:2388–2397
Giorgi D, Anni M (2019) Amplified spontaneous emission and lasing in lead halide perovskites: state of the art and perspectives. Appl Sci 9(21):4591
Guo HY, Pei Y, Zhang J, Cai C, Zhou K, Zhu Y, (2019) Doping with SnBr2 in CsPbBr3 to enhance the efficiency of all-inorganic perovskite solar cells, J Mater Chem C 11234-11243
Hu G, Qin W, Liu M, Ren X, Wu X, Yang L, Yin S (2019) Scalable room-temperature synthesis of plum-pudding-like Cs4PbBr6/CsPbBr3 microcrystals exhibiting excellent PL. J Mater Chem C 7:4733–4739
Jia C, Li H, Meng X (2018) CsPbX3/Cs4PbX6 core/shell perovskite nanocrystals. Chem Commun 54:6300–6303
Jing Q, Xu Y, Su Y, Xing X, Lu Z (2019) A systematic study of the synthesis of cesium lead halide nanocrystals: does Cs4PbBr6 or CsPbBr3 form. Nanoscale 11:1784–1789
Jones M, Lo SS, Scholes GD (2009) Signatures of exciton dynamics and carrier trapping in the time-resolved PL of colloidal CdSe nanocrystals. J Phys Chem C 43:18632–18642
Kang B, Biswas K (2018) Exploring polaronic excitonic structures and luminescence in Cs4PbBr6/CsPbBr3. J Phys Chem Lett 9:830–836
Li Z, Kong L, Huang SQ, Li L (2017) Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/alumina monolith. Angew Chem Int Ed 56:8134–8138
Li M, Zhang X, Matras-Postolek K, Chen HS, Yang P (2018a) An anion-driven Sn2+ exchange reaction in CsPbBr3 nanocrystals towards tunable and high photoluminescence. J Mater Chem C 6:5506–5513
Li Y, Huang H, Xiong Y, Kershaw SV, Rogach AL (2018b) Reversible transformation between CsPbBr3 and Cs4PbBr6 nanocrystals, RSC CrystEngComm 4900–4904, 20
Ling Y, Tan L, Wang X, Zhou Y, Xin Y, Ma B, Hanson K, Gao H (2017) Composite perovskites of cesium lead bromide for optimized PL. J Phys Chem Lett 8:3266–3271
Liu S, Shao G, Ding L, Liu J, Xiang W, Liang X (2019) Sn-doped CsPbBr3 QDs glasses with excellent stability and optical properties for WLED. Chem Eng J 361:937–944
Lou S, Zhou Z, Xuan TT, Li H, Zhang H, Gautier R, Wang J (2019) Chemical transformation of lead halide perovskite into insoluble less cytotoxic and brightly luminescent CsPbBr3/CsPb2Br5 composite nanocrystals for cell imaging. ACS Appl Mater Interfaces 27:24241–24246
Mello Donegá C, Bode M, Meijerink A (2006) Size-and temperature-dependence of exciton lifetimes in CdSe quantum dots. Phys Rev 8:085320
Nedelcu G, Protesescu L, Yakunin S, Bodnarchuk MI, Grotevent MJ, Kovalenko MV (2015) Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett 15:5635–5640
Quan LN, Quintero-Bermudez R, Voznyy O, Walters G, Jain A, Fan JZ, Zheng X, Yang Z, Sargent EH (2017) Highly emissive green perovskite nanocrystals in a solid crystalline matrix. Adv Mater 29:1605945
Riesen N, Lockrey M, Badek K, Riesen H (2018) On the origins of the green luminescence in the zero-dimensional perovskite Cs4PbBr6: conclusive results from cathodoluminescence imaging. Nanoscale. 00:1–3
Saidaminov MI, Almutlaq J, Sarmah S, Dursun I, Zhumekenov AA, Begum R, Pan J, Cho N, Mohammed OF, Bakr OM (2016) Pure Cs4PbBr6: highly luminescent zero-dimensional perovskite solids. ACS Energy Lett 1:840–845
Saliba M, Correa-Baena JP, Hagfeldt A, Abate A (2018) Perovskite solar cells: from the atomic level to film quality and device performance. Angew Chem Int Ed 57:2554–2569
Shen Z, Qiao B, Xu Z, Song D, Gao D, Song P, Cao J, Bai Q, Wu Y, Zhao S, (2019) The luminescence properties of CsPbxM1−xBr3 perovskite nanocrystals transformed from Cs4PbBr6 mediated by various divalent bromide MBr2 salts, Nanoscale. 4008-4014
Su M, Fan B, Li H, Wang K, Lou ZK (2019a) Hydroxyl terminated mesoporous silica-assisted dispersion of ligand-free CsPbBr3/Cs4PbBr6 nanocrystals in polymer for stable white LED, Nanoscale 1335–1342
Su Y, Zeng Q, Chen X, Ye W, She L, Gao X, Ren Z, Li X (2019b) Highly efficient CsPbBr3 perovskite nanocrystals induced by structure transformation between CsPbBr3 and Cs4PbBr6 phases, J Mater Chem C 7548-7533
Sun C, Gao Z, Liu H, Wang L, Deng Y, Zhang ZH, Fan C, Bi W (2019) One stone, two birds: high-efficiency blue-emitting perovskite nanocrystals for LED and security ink applications. Chem Mater 14:5116–5123
Wang Y, Yu D, Wang Z, Li X, Chen X, Nalla V, Zeng H, Sun H (2017) Solution grown CsPbBr3/Cs4PbBr6 perovskite nanocomposites: toward temperature insensitive optical gain. Small 13:1701587
Wei S, Yang Y, Kang X, Wang L, Huang L, Pan D, (2016) Room-temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50–85% PLQYs, Chem Commun 7265–7268
Wu L, Hu H, Xu Y, Jiang S, Chen M, Zhong Q, Yang D, Liu Q, Zhao Y, Sun B, Zhang Q, Yin Y (2017) From nonluminescent Cs4PbX6(X=Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: water-triggered transformation through a CsX-stripping mechanism. Nano Lett 17:5799–5804
Xu L, Li J, Fang T, Zhao Y, Yuan S, Dong Y, Song J (2019) Synthesis of stable and phase-adjustable CsPbBr3 @Cs4PbBr6 nanocrystals via novel anion–cation reactions. Nanoscale Adv 1:980–988
Xuan T, Lou S, Huang J, Cao L, Yang X, Li H, Wang J (2018) Monodisperse and brightly luminescent CsPbBr3/Cs4PbBr6 perovskite composite nanocrystals, RSC Nanoscale 10: 9840–9844
Zhang X, Sun C, Zhang Y, Wu H, Ji C, Wang P, Wen S, Zhang C, Yu WW (2016) Bright perovskite nanocrystal films for efficient light-emitting devices, J Phys Chem Lett 4602–4610
Zou S, Liu C, Li R, Jiang F, Chen X, Liu Y, Hong M (2019) From nonluminescent to blue-emitting Cs4PbBr6 nanocrystals: tailoring the insulator bandgap of 0D perovskite through Sn cation doping. Adv Mater 31:1900606
Funding
This work was supported in part by the projects from the National Natural Science Foundation of China (no. 51772130 and 51972145) and the Independent Innovation Team from Ji Nan Science & Technology Bureau (grant no. 2019GXRC016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Li, M., Matras-Postolek, K. et al. Crystal structure and luminescence of Cs-Pb-Sn-Br nanocrystals. J Nanopart Res 22, 291 (2020). https://doi.org/10.1007/s11051-020-05020-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-05020-4