Abstract
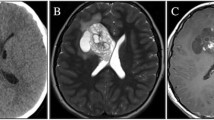
The neural basis for epilepsy and attention deficit hyperactivity disorder (ADHD) is currently incompletely known. We reported a young girl with both epilepsy and ADHD, who had a calcified lesion in the right basolateral amygdalo-hippocampal region extending to the ventral striatum. The child underwent disconnecting surgery and biopsy of the lesion. Fascinatingly, the child’s behavior changed immediately after the surgery from inattentive and impulsive to nearly normal behavior experiencing no more breakthrough seizures since after 3 years of surgery. The Schaltenbrand Wahren Brain Atlas revealed alveus, cornu ammonis, amygdala superficialis, and medium as the disconnected region in this surgery.



Similar content being viewed by others
References
Kanner AM, Scharfman H, Jette N, Anagnostou E, Bernard C, Camfield C, Camfield P, Legg K, Dinstein I, Giacobbe P, Friedman A, Pohlmann-Eden B (2017) Epilepsy as a network disorder (1): what can we learn from other network disorders such as autistic spectrum disorder and mood disorders? Epilepsy Behav 77:106–113
Socanski D, Aurlien D, Herigstad A, Thomsen PH, Larsen TK (2013) Epilepsy in a large cohort of children diagnosed with attention deficit/hyperactivity disorders (ADHD). Seizure 22(8):651–655
Vidaurre J, Twanow JDE (2017) Attention deficit hyperactivity disorder and associated cognitive dysfunction in pediatric epilepsy. Semin Pediatr Neurol 24(4):282–291
Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS (2006) Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63(7):795–807
Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal JD, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL (2001) Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 58(3):289–295
Silk TJ, Vilgis V, Adamson C, Chen J, Smit L, Vance A, Bellgrove MA (2016) Abnormal asymmetry in frontostriatal white matter in children with attention deficit hyperactivity disorder. Brain Imaging Behav 10(4):1080–1089
van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Oosterlaan J (2014) Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry 53(7):790–799.e793
Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, Peterson BS (2011) Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50(8):828–837.e823
Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K (2010) Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res 44(10):629–639
Smith AB, Taylor E, Brammer M, Toone B, Rubia K (2006) Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 163(6):1044–1051
Aman CJ, Roberts RJ, Pennington BF (1998) A neuropsychological examination of the underlying deficit in attention deficit hyperactivity disorder: frontal lobe versus right parietal lobe theories. Dev Psychol 34(5):956–969
Howes OD, Rogdaki M, Findon JL, Wichers RH, Charman T, King BH, Loth E, McAlonan GM, McCracken JT, Parr JR, Povey C, Santosh P, Wallace S, Simonoff E, Murphy DG (2018) Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J Psychopharmacol 32(1):3–29
Oades RD (1998) Frontal, temporal and lateralized brain function in children with attention-deficit hyperactivity disorder: a psychophysiological and neuropsychological viewpoint on development. Behav Brain Res 94(1):83–95
Yu G, Sharp BM (2015) Basolateral amygdala and ventral hippocampus in stress-induced amplification of nicotine self-administration during reacquisition in rat. Psychopharmacology 232(15):2741–2749
Schaltenbrand G, Wahren W (1998) Atlas for Stereotaxy of the human brain. Thieme, Stuttgart
Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N, Zeeuw P, Szekely E, Sudre G, Wolfers T, Onnink AMH, Dammers JT, Mostert JC, Vives-Gilabert Y, Kohls G, Oberwelland E, Seitz J, Schulte-Rüther M, Ambrosino S, Doyle AE, Høvik MF, Dramsdahl M, Tamm L, van Erp TGM, Dale A, Schork A, Conzelmann A, Zierhut K, Baur R, McCarthy H, Yoncheva YN, Cubillo A, Chantiluke K, Mehta MA, Paloyelis Y, Hohmann S, Baumeister S, Bramati I, Mattos P, Tovar-Moll F, Douglas P, Banaschewski T, Brandeis D, Kuntsi J, Asherson P, Rubia K, Kelly C, Martino AD, Milham MP, Castellanos FX, Frodl T, Zentis M, Lesch KP, Reif A, Pauli P, Jernigan TL, Haavik J, Plessen KJ, Lundervold AJ, Hugdahl K, Seidman LJ, Biederman J, Rommelse N, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Oosterlaan J, Polier GV, Konrad K, Vilarroya O, Ramos-Quiroga JA, Soliva JC, Durston S, Buitelaar JK, Faraone SV, Shaw P, Thompson PM, Franke B (2017) Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4(4):310–319
Park HR, Kim IH, Kang H, Lee DS, Kim BN, Kim DG, Paek SH (2017) Nucleus accumbens deep brain stimulation for a patient with self-injurious behavior and autism spectrum disorder: functional and structural changes of the brain: report of a case and review of literature. Acta Neurochir 159(1):137–143
Sinha S, McGovern RA, Sheth SA (2015) Deep brain stimulation for severe autism: from pathophysiology to procedure. Neurosurg Focus 38(6):E3
Sturm V, Fricke O, Buhrle CP, Lenartz D, Maarouf M, Treuer H, Mai JK, Lehmkuhl G (2012) DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci 6:341
Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M (2014) Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum Brain Mapp 35(9):4693–4705
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The video shows patient’s condition at preoperative period, at seven day and one year after the surgery. Noteworthy that marked improvement was immediately noted after the resective-disconnecting surgery. (WMV 191049 kb)
Rights and permissions
About this article
Cite this article
Idris, Z., Zakaria, Z., Halim, S.A. et al. Disconnecting surgery at alveus and cornu ammonis of hippocampus, amygdala superficialis, and amygdala medial nuclei for epilepsy associated with attention deficit hyperactivity disorder. Childs Nerv Syst 37, 1797–1802 (2021). https://doi.org/10.1007/s00381-020-04893-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04893-z