Abstract
In this work, the influence of initial viscosity on the Trommsdorff effect was firstly investigated, and it was confirmed that 1000 ± 100 Pa · s of viscosity could be a criterion of the Trommsdorff effect. On this basis, a new inverted two-stage extruder was established, the designed 'blocking unit' was introduced to the screw configuration, and then the continuous bulk copolymerization of methyl methacrylate (MMA) and styrene (St) was carried out. The results showed that the blocking unit could effectively enhance the residence time of copolymerization, and make the molecular weight distribution of copolymer more uniform. The position of the Trommsdorff effect in the extruder was then determined by the thermal effect of copolymerization, and the mixed monomers were continuously added to effectively utilize the Trommsdorff effect. As a result, the MMA-St copolymers with high molecular weight and narrow distribution were prepared via reactive extrusion, which possessed higher melt flow rate and better thermal stability than pure-PMMA. The present work provides important reference and guidance for the industrialization of bulk free radical copolymerization via reactive extrusion.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
In recent decades, the reactive extrusion technology using extruder as the continuous polymerization reactor has developed rapidly [1, 2]. Compared with the conventional reactor, the unique advantage of extruder is its high-efficiency mass and heat transfer for high-viscosity system. In addition, reactive extrusion combines the synthesis and molding of polymers into a continuous process, to obtain the target materials in one step, and almost no solvent is used. Therefore, significant improvement of polymerization efficiency and cost reduction could be achieved, energy consumption and pollution are also decreased. As more and more attention has been paid to energy conservation and environmental protection [3–6], the above superiorities of reactive extrusion polymerization make it become a clean technology.
The residence time in a common extruder is within 30 min, hence the anionic polymerization is widely applied to reactive extrusion due to its high polymerization rate (3–10 min) [7, 8]. For example, Michaeli et al [9, 10] had prepared polystyrene by bulk anionic polymerization using a co-rotating twin-screw extruder as the reactor, and n-butyllithium was used as the initiator. Our previous work [11–16] had investigated the bulk anionic polymerization and bulk anionic ring-opening polymerization via reactive extrusion for more than decade, a series of styrene-diene copolymer/rubbers and fluorosilicone rubber were successfully synthesized. However, although free radical polymerization is extensively applied in industry, it's still very difficult to carry out by reactive extrusion due to its slow polymerization rate (such as several hours).
Berthet et al [17] had investigated the bulk free radical polymerization of n-butyl methacrylate (BMA) in a twin-screw extruder. They combined the free volume theory [18, 19] with the kinetic model, making the polymerization system adapt to the short residence time in extruder, and found the optimal polymerization conditions. The results showed that the temperature and screw speed must be kept at low value to prolong the polymerization time, as a result the conversion could reach to 98%, but the yield was significantly limited. Janssen et al [20–22] had reported the homo-polymerization process of BMA and its copolymerization with 2-hydroxypropyl methacrylate in a twin-screw extruder. The results showed that the polymerization kinetic had significant impact on the process of reactive extrusion. The early occurrence of Trommsdorff effect [23, 24] could effectively improve the conversion and molecular weight of resultant polymers.
In order to accomplish the free radical polymerization within a short residence time in extruder, the polymerization rate could be improved by increasing the temperature or the initiator dosage, which would always lead to the significantly decline of molecular weight of polymer [25]. Moreover, the yield is also dramatically reduced under lower screw speed, limiting the industrialization of reactive extrusion polymerization [17]. We suggest that effectively utilizing the Trommsdorff effect is perhaps a method to realize the simultaneous improvement of polymerization rate and molecular weight.
The Trommsdorff effect is generally thought to be induced by the diffusion limitation of chain termination [26], resulting in the significant acceleration of polymerization and the increase of chain length. For traditional bulk polymerization methods [27], the Trommsdorff effect must be strictly avoided or controlled due to the intense heat release within a short time. However, those problems could be well solved while the polymerization is conducted in an extruder. On the contrary, if the Trommsdorff effect could be fully utilized during the reactive extrusion process, then the continuous bulk polymerization with high polymerization rate as well as high molecular weight could be realized.
In this work, the relationship between the Trommsdorff effect and viscosity was verified, and the methyl methacrylate-styrene copolymers (MMA-St copolymers) were successfully prepared by an inverted two-stage extruder based on utilizing the Trommsdorff effect, which represented an important step for the industrialization of free radical copolymerization by reactive extrusion.
Experimental
Materials
MMA and St, analytical grade, was provided by China's Oil Sunup Group Co., LTD, and purified by vacuum distillation prior to use. Benzoyl peroxide (BPO), azobisisobutyronitrile (AIBN) and dicumyl peroxide (DCP), analytical grade, were supplied by Shanghai Lingfeng Chemical Reagent Co., Ltd. BPO was refined by dissolving in chloroform, then precipitating by cold methanol. Homo-PMMA (PMMA-C, Mn = 4.3 × 104, MWD = 1.61), was purchased from Mitsubishi Rayon Polymer Nantong Co., Ltd.
Research on the Trommsdorff effect under different initial viscosity
PMMA-C was firstly predissolved in MMA with a mass fraction of 25 wt%, and obtained the PMMA/MMA solution. Subsequently, 0.3 wt% of BPO was added to PMMA/MMA solution and pure MMA, respectively, and obtained the polymerization solution. The temperature of the SU-70B Torque Rheometer (Changzhou Suyan Technology Co., Ltd, China) was preheated to 90 ℃, and the rotating speed was set at 20 rpm. The polymerization solution was then fed into the Torque Rheometer, recorded the change of torque and temperature with time until the torque was above 50 N·m.
In order to further investigate the relationship between Trommsdorff effect and initial viscosity, the different PMMA/MMA solutions were obtained by predissolving with PMMA-C at a mass fraction of 5 wt%, 10 wt%, 20 wt%, and 30 wt%, respectively. The viscosity of PMMA/MMA solutions were measured at 0.1 Hz by the RS600 Haake Rotational Rheometer (Thermo Haake Co., Germany). The polymerization of PMMA/MMA solutions were carried out by the Modulated DSC 291 Differential Scanning Calorimeter (TA Instruments Co., USA), the concentration of BPO was 0.5 wt% and the temperature was constant at 90 ℃. The polymerization rate curves were obtained by differentiating the exotherm curves.
Establishment of the inverted two-stage extruder
The common extruders are usually fed from the root of the screw (near the gearbox) and the product is extruded through the die. However, the reactant for reactive extrusion polymerization is monomers with extremely low viscosity, they are easy to leak out after being fed into the extruder. Moreover, the forward conveying of low-viscosity monomers in extruder is also a problem. In this study, we designed a new extruder structure of 'inverted screw', as shown in figure 1.
Figure 1. Experimental setup for reactive extrusion of MMA-St copolymer. 1. TDE-40 extruder 2.TDY-40 extruder 3. Connector 4, 5, 6. Metering pumps 7. Vacuum pump.
Download figure:
Standard image High-resolution imageTDE-40 co-rotating closely intermeshing twin-screw extruder (D = 41.3 mm, L/D = 68, Useon Extrusion Machinery Co., Ltd, China) and TDY-40 counter-rotating closely intermeshing twin-screw extruder (D = 41 mm, L/D = 60, Useon Extrusion Machinery Co., Ltd, China) were reversely connected through a connector, to effectively increase the residence time. The die of the first-stage extruder (TDE-40) was sealed, and the monomers were fed from the head of the first-stage extruder. The rotation direction of the screws was reverse to the normal situation. The viscosity of reactant will rise to a certain value while conveyed to the root of the first-stage extruder, so the leakage of monomers could be effectively prevented. The head of the second-stage extruder (TDY-40) was connected to the first stage extruder, and the vacuum devolatilization device was set at the tenth barrel, and the die was set at the eleventh barrel near the gearbox. The feeding system contained three high-pressure metering pumps (Hangzhou Zhijiang Petrochemical Equipment Co., China), which could ensure the forward conveying of monomers through continuous pressure.
As the initial viscosity of MMA or St is close to 1 cP, they were hard to adhere to the screw units. Therefore, the residence time of monomers on every screw unit was very short, resulting in the waste of effective region of screw. In this study, we introduced a new screw unit named 'blocking unit' into the first-stage extruder, not just the traditional conveying units and kneading blocks. The structure diagram of the blocking unit is shown in figure 2, which is composed of two disks with different diameters. The function of this blocking unit is to prevent most of the monomers with low viscosity from flowing through the screw units, hence the residence time of polymerization system could be greatly enhanced.
Figure 2. Schematic diagram of the blocking unit.
Download figure:
Standard image High-resolution imageSynthesis of MMA-St copolymers via reactive extrusion
The inverted two-stage extruder was used as the polymerization reactor, AIBN and DCP were used as the low-temperature initiator and high-temperature initiator, respectively. The temperature setting was divided into low-temperature zone, temperature-incremental zone and high-temperature zone. The polymerization was mainly completed in the first two zones, and the melt transportation was conducted in the high-temperature zone. The mixed monomers were firstly added to tank A, B and C, respectively. Then a specific ratio of AIBN was dissolved in the solution in tank A and tank B, a specific ratio of DCP was dissolved in the solution in tank C, the ratios of monomer and initiator are presented in table 1. The extruder was preheated to 200 ℃, and purged by dry argon to remove impurities before polymerization.
Table 1. Monomer ratios and initiator contents.
| Initiator dose (wt%) | ||||||
|---|---|---|---|---|---|---|
| Experiments | Samples | MMA (wt%) | St (wt%) | Tank A | Tank B | Tank C |
| 1 | P(MMA-co-St)-M | 90 | 10 | 0.4 | / | / |
| 2 | P(MMA-co-St)-N | 90 | 10 | 0.4 | / | / |
| 3 | P(MMA-co-St)-5 | 95 | 5 | 0.4 | 0.1 | 0.1 |
| 4 | P(MMA-co-St)-10 | 90 | 10 | 0.4 | 0.1 | 0.1 |
| 5 | P(MMA-co-St)-20 | 80 | 20 | 0.4 | 0.1 | 0.1 |
In order to determine the position of Trommsdorff effect in the first-stage extruder, pump 5 and pump 6 were turned off, and the mixture in tank A was pumped into the feeding inlet of the first-stage extruder at a rate of 5 l· h−1, while the screw speed was gradually increased to the set value. The reactant formula was based on Experiment 1 in table 1. When the reactive extrusion process was stable, the cooling system of the first-stage extruder was turned off, and recorded the temperature change of each barrel. The copolymer prepared by once feeding was named as P(MMA-co-St)-M. In order to investigate the influence of blocking unit on the copolymerization process, the copolymerization experiment was carried out while the blocking units weren't introduced to the first-stage extruder, with the other conditions remaining unchanged. The obtained copolymer was named as P(MMA-co-St)-N.
After the once-feeding polymerization was stable, pump 5 and pump 6 were turned on in sequence, the mixture in tank B and tank C was added to the thirteenth barrel of the first-stage extruder and the second barrel of the second-stage extruder at the feeding rates of 2 l· h−1, respectively, as shown in figure 1. The residual monomers were removed by the devolatilization device, and the P(MMA-co-St) with different content of St was obtained after pelletizing process. The copolymers were named as P(MMA-co-St)-5, P(MMA-co-St)-10 and P(MMA-co-St)-20, respectively.
Multi-detector gel permeation chromatography (MGPC) analysis
The molecular weight and distribution of P(MMA-co-St) was characterized by Water 515 MGPC spectrometer (Wyatt Technology Corporation, USA), connected with a refractive detector (RI signal) and a small-angle light scattering detector (LS signal). THF was used as the eluent at a flow rate of 1.0 ml· min−1, and the test was conducted at 25 ℃.
Proton nuclear magnetic resonance (1H-NMR) analysis
The molecular structure of P(MMA-co-St) was analyzed by DRX-400 1H-NMR spectrometer (Bruker Corporation, Germany) at room temperature. The tetramethylsilane and deuterated chloroform was used as the internal standard and the solvent, respectively.
Melt flow rate analysis
The melt flow rate of the copolymer was determined by ZRZ1452 melt flow rate tester, according to the GB/T 3682-2000 (China), with the testing temperature of 230 ℃ and the load weight of 3.8 kg.
Thermogravimetric analysis (TGA)
The thermal stability of the copolymer was characterized by TGA 800 thermogravimetric analyzer (Perkin Elmer Corporation, USA). The temperature ranged from 40 ℃ to 600 ℃ at 10 K· min−1 in nitrogen atmosphere.
Mechanical performances analysis
The copolymers were molded by plastic injection. The injection pressure was set at 65 MPa to 80 MPa, the mold temperature was 50 ℃, and the plasticizing temperature ranged from 190 ℃ to 230 ℃. The tensile and flexural strengths were measured through a CMT4204 universal material testing machine with the test rate of 5 mm· min−1, the test sticks were the shape of dumbbell with the dimension of Type A according to the standard ISO 3167: 2002, and each sample was tested for five times. The impact strength was characterized by a CEAST 9050 cantilever beam impact tester with the test rate of 2 mm· min−1, the sample size was 80 × 10 × 4 mm according to the standard ISO 8257-2: 2001, and each sample was tested for five times.
Results and discussion
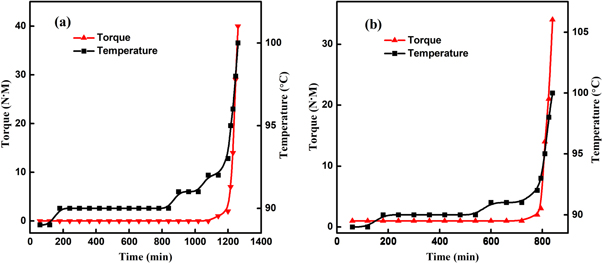
The bulk polymerization of MMA was carried out in the Torque Rheometer, and the torque and temperature curves are shown in figure 3(a). It can be seen that the torque of the polymerization system was almost constant before 1080 s. As the polymerization proceeded, the torque and temperature both rose dramatically after 1200 s, indicating the autoacceleration of the polymerization. The saltation point of torque was regarded as the sign when the Trommsdorff effect appeared. Under the same experimental conditions, the torque and temperature curves of the polymerization of MMA solution predissolved with PMMA (25 wt%) were shown in figure 3(b). It can be found that the saltation time of torque was at 780 s, which was 420 s earlier than the pure-MMA polymerization. This indicated that the occurrence of Trommsdorff effect was closely related to the initial viscosity of the polymerization system, and the Trommsdorff effect would occur earlier if the initial viscosity was increased. Then, as the initial viscosity increases gradually, will there be a critical viscosity, at which the Trommsdorff effect would appear?
Figure 3. Torque & temperature versus time curves. (a) pure MMA system. (b) PMMA /MMA system.
Download figure:
Standard image High-resolution imageIn our previous work, the kinetics of bulk polymerization of MMA was comprehensively investigated [28]. The results showed that the viscosity of polymerization solution (measured at 0.1 Hz) when the Trommsdorff effect occurred was within the range of 1000 ± 100 Pa · s. Moreover, the viscosity variation of bulk polymerization of MMA was detected online by the Rotational Rheometer [29]. It was found that the critical viscosities were quite close under different shear frequencies, which approximated to 1000 Pa · s, and in accordance with the critical viscosity obtained by kinetics reaearch [28]. In order to verify this critical viscosity, the PMMA/MMA solutions with different concentrations were firstly prepared in this work, and the viscosities of PMMA/MMA solutions were measured at 0.1 Hz, as shown in table 2. Subsequently, the polymerization of different PMMA/MMA solutions was carried out in DSC, and the exothermic curves were shown in figure 4.
Table 2. Viscosity of PMMA/MMA solution at 0.1 Hz.
| Mass fraction | 0 | 5% | 10% | 20% | 30% |
| Viscosity (Pa · s) | 0 | 79 | 425 | 894 | 1783 |
Figure 4. DSC curves of polymerization of PMMA/MMA solution with different concentrations.
Download figure:
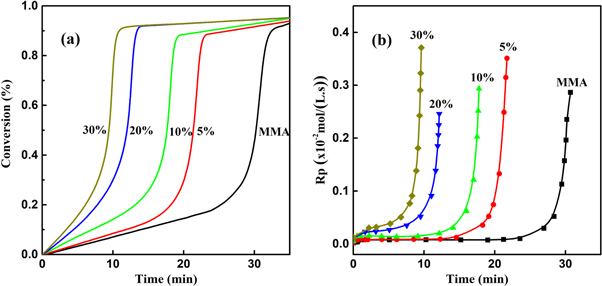
Standard image High-resolution imageIt can be seen that as the mass fraction of PMMA (namely the initial viscosity) increased, the onset points of the exothermic peaks appeared forward, and the time of the maximum exothermic peak was at 31 min, 22 min, 18 min, 13 min, and 10 min, respectively. This indicated that the polymerization rate would increase significantly as the viscosity increased, resulting to the early occurrence of Trommsdorff effect. The DSC curve was subsequently differentiated and normalized, and obtained the conversion curves and the polymerization rate curves, as shown in figure 5.
Figure 5. Conversion-time curve (a) and polymerization rate-time curve (b) for PMMA/MMA solutions with different polymer contents.
Download figure:
Standard image High-resolution imageAccording to figure 5(a), the conversion curves present the shape of 'S', which were consistent with the typical bulk free radical polymerization. For the polymerization of pure MMA and the one with 5 wt% of predissolved PMMA (initial viscosity of 79 Pa · s), there were obvious periods with constant polymerization rate before the Trommsdorff effect, and the polymerization rates at this 'quasi-steady state' were both 1 × 10−3 mol/(l· s), as shown in figure 5(b). The onset times of the Trommsdorff effect for the two systems were at 24 min and 15 min, respectively. For the polymerization with an initial viscosity of 425 Pa · s (10 wt% of PMMA), the polymerization rate at the 'quasi-steady state' reached to 3 × 103 mol/(l · s) due to the increase of viscosity, and the Trommsdorff effect appeared at 10 min. When the initial viscosity increased to 894 Pa · s (closed to 1000 ± 100 Pa · s), the polymerization rapidly got into the Trommsdorff effect region after a transient 'quasi-steady state'. When the initial viscosity reached to 1783 Pa·s (far above 1000 Pa · s), the polymerization curves had almost no 'quasi-steady state', presenting the character of autoacceleration at the beginning of polymerization. The above results proved that the occurrence of Trommddorff effect could be controlled by regulating the initial viscosity of polymerization solution, and the viscosity range of 1000 ± 100 Pa · s could be regarded as an effective criterion for the occurrence of Trommsdorff effect, which provided a theoretical foundation for controlling and utilizing the Trommsdorff effect.
Trommddorff effect in the inverted two-stage extruder
The first-stage extruder consisted of 8 temperature-control zones, each temperature-control zone consisted of 2 barrels. When the reactive extrusion copolymerization by once feeding was stable, the cooling system was firstly turned off, and then recorded the temperature variation of each zone, the result was shown in figure 6. When the cooling system was running, the circulating water would quickly remove the heat generated by the copolymerization, so the temperature of the first-stage extruder was stable at 90 ℃. However, the temperature of each zone rose distinctly when the cooling system was turned off, and the temperature rise was incremental along the transportation direction.
Figure 6. Temperature variation curves of the first-stage extruder along the transportation direction.
Download figure:
Standard image High-resolution imageAs the room-temperature MMA/St monomers were continuously fed into the extruder, and a short induction period existed in the early stage of copolymerization, the temperature of the first temperature-control zone kept unchanged. And the temperature rose tardily in the second, third and fourth temperature-control zones, mainly attributing to the low polymerization rate at the 'quasi-steady state'. However, the temperature began to rise rapidly from the sixth temperature-control zone, and then significantly increased in the seventh temperature-control zone with a temperature rise of 12 ℃. This indicated that the Trommsdorff effect of copolymerization of MMA and St had occurred in the seventh zone, with significant decline of chain termination rate constant (kt) due to the increase of viscosity, and leading to the release of massive heat within a short time. As a result, the new MMA/St monomers could be added to the corresponding thirteenth barrel, and the consecutive copolymerization with high polymerization rate and high molecular weight of resultant polymer could be realized.
Influence of blocking unit on the reactive extrusion copolymerization
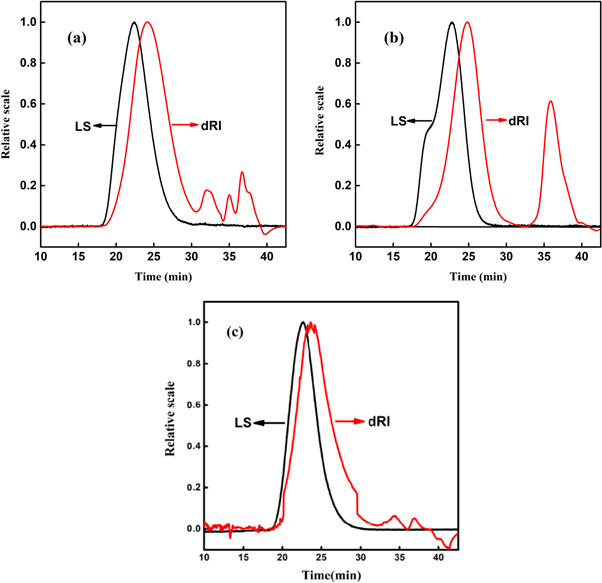
Figures 7(a) and (b) are the GPC curves of the samples prepared while introducing the blocking unit (P(MMA-co-St)-M) and not introducing the blocking unit (P(MMA-co-St)-N) by once feeding, the LS signal was sensitive to the high molecular weight components and the dRI signal was sensitive to the low molecular weight components. It can be seen that the dRI curve of P(MMA-co-St)-M shows a group of smaller peaks within the elution time of 30–40 min, with corresponding molecular weight of 8000. However, the GPC curve of P(MMA-co-St)-N presents a typical bimodal distribution, with a high-intensity peak near the elution time of 35 min. When the blocking unit was not introduced to the screw of the first-stage extruder, the polymerization system was quickly transported to the high-temperature zone (over 100 ℃), and a large amount of AIBN as well as unreacted monomers were still residual in this position. Therefore, a lot of AIBN was decomposed rapidly under the high temperature, initiating the copolymerization and generating many low molecular weight components. In contrast, while the blocking unit was introduced, the residence time in the first-stage extruder significantly increased, resulting the obvious reduction of the low molecular weight component. Moreover, the distribution of the peak with low molecular weight was also more dispersed and complicated for P(MMA-co-St)-M, indicating that the monomers was not copolymerized intensively at a specific position. According to the data in table 3, the molecular weight distribution (MWD) of P(MMA-co-St)-M was much smaller than that of P(MMA-co-St)-N, and with a higher copolymerization conversion. The above results indicated that introducing the blocking unit could effectively improve the residence time in the extruder, and make the copolymerization process more uniform.
Figure 7. GPC curves of copolymers (a) P(MMA-co-St)-M, (b) P(MMA-co-St)-N and (c) P(MMA-co-St)-10.
Download figure:
Standard image High-resolution imageTable 3. Parameters of the reactive extrusion copolymerization of different samples.
| Samples | Conversions (%) | Residual rates of monomers (%) | Mn × 10−4 | Mw × 10−4 | MWD |
|---|---|---|---|---|---|
| P(MMA-co-St)-M | 98.6 | 0.05 | 4.0 | 7.9 | 1.98 |
| P(MMA-co-St)-N | 90.1 | 0.45 | 4.3 | 9.5 | 2.25 |
| P(MMA-co-St)-5 | 98.0 | 0.07 | 6.8 | 10.9 | 1.60 |
| P(MMA-co-St)-10 | 98.5 | 0.05 | 6.8 | 11.1 | 1.65 |
| P(MMA-co-St)-20 | 98.2 | 0.04 | 6.7 | 11.5 | 1.68 |
Compared with P(MMA-co-St)-M synthesized via once feeding, the GPC curve of P(MMA-co-St)-10 synthesized via thrice feeding presents a typical unimodal distribution, and the intensity of peak within 30–40 min is very small, as shown in figure 7(c). This is due to that the twice added monomers would not only copolymerize with high polymerization rate, but also generate copolymer with high molecular weight, as a result the content of high molecular weight component in the copolymer relatively increased. Moreover, more monomers would participate in the copolymerization at the high-temperature zone while the monomers were added, which would make the content of low molecular weight components relatively reduced, and then make the MWD more uniform. According to the data in table 3, the Mn of the copolymers with different St contents were all above 6.7 × 104, increased by more than 2 × 104 compared with P(MMA-co-St)-M, and the MWD was also significantly narrowed. This results indicated that, P(MMA-co-St) with high molecular weight and narrow distribution could be prepared by effectively utilizing the Trommsdorff effect via reactive extrusion.
Molecular structure of P(MMA-co-St)
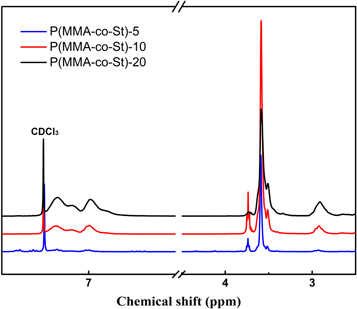
Figure 8 shows the 1H-NMR spectrum of P(MMA-co-St). The proton peaks of C=C in MMA and St didn't appear at δ = 5.55 and δ=6.09, indicating that there were no residual monomers in the copolymer. The absorption bands between 6.82 ∼ 7.24 ppm corresponded to the protons on the benzene ring and represented the characteristic peak of the St unit. The signal at 2.90 ppm belonged to the proton peak of –CH connected to the phenyl. It can be seen that as the ratio of St increased, the intensity of the proton peak of the St unit gradually increased. On the other hand, the absorption bands from 3.40 to 3.78 belonged to the methyl protons of –COOCH3, which represented the characteristic peak of the MMA unit in the copolymer. The content of St unit in P(MMA-co-St) could be calculated by the ratio of the peak area of phenyl protons (6.82 ∼ 7.24 ppm) to the peak area of –COOCH3 (3.40 ∼ 3.78 ppm), and the results were 4.95%, 10.06% and 19.98%, respectively, which was almost consistent with the feeding ratio. This result indicated that the copolymerization of MMA and St had been successfully conducted by reactive extrusion, and the conversion of monomers was nearly complete. As the reactivity ratios of MMA and St is both about 0.5 [30], the two monomers tend to participate in the copolymerization with the similar probability. As a result, under the high-shear environment in extruder, the content of St in the copolymer tended to be the same as the feeding ratio of monomers, and more uniformly distributed in the molecular chain.
Figure 8. 1H-NMR spectrum of P(MMA-co-St).
Download figure:
Standard image High-resolution imageRheological analysis of P(MMA-co-St)
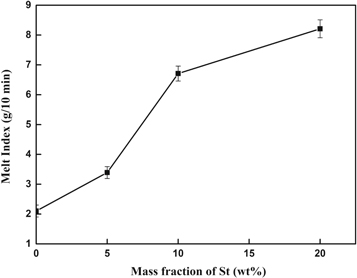
PMMA is a kind of polar polymer with high melt viscosity, so St units were introduced to improve the melt fluidity of PMMA in this study. The melt flow rate curve of P(MMA-co-St) at different ratios is shown in figure 9. It can be seen that although the molecular weight of P(MMA-co-St) was 2 × 104 higher than that of PMMA-C, the melt flow rate of P(MMA-co-St) was significantly increased with the increase of St content, and reached to 3.4, 6.7 and 8.2 g/(10 min), respectively. This was due to that the introduction of non-polar St unit would effectively weaken the force between the polar chains of PMMA, making the movement of the chains much easier, hence the melt viscosity of the prepared P(MMA-co-St) was greatly reduced compared to the pure-PMMA. As a result, the introduction of St units could significantly improve the melt fluidity and the processing performance of PMMA, and the warpage caused by residual stress could be consequently avoided, which is beneficial to expand the application of PMMA in large sheet, LCD screen and so on.
Figure 9. Melt flow rate curve of P(MMA-co-St).
Download figure:
Standard image High-resolution imageThermal stability of P(MMA-co-St)
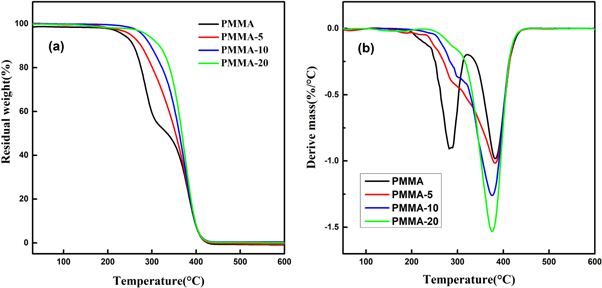
Figure 10(a) presents the TGA curves of P(MMA-co-St). The initial decomposition temperature and the temperature with 50 wt% of weight loss are marked as Tonset and Td50, respectively. And the corresponding data of Tonset and Td50 are listed in table 4. It can be seen that the Tonset of P(MMA-co-St) increased by 9 ℃, 26 ℃ and 37 ℃ compared with pure-PMMA, and the Td50 increased by 16 ℃, 25 ℃ and 32 ℃, respectively. This indicated that the thermal stability of PMMA could be significantly enhanced by introducing St units. Moreover, it can be seen from figure 10(b) that the DTG curve of PMMA shows two obvious peaks of maximum weight-loss, while the DTG curves of P(MMA-co-St) present only one peak of maximum weight-loss. As the degradation of PMMA under lower temperature (below 300 ℃) is mainly initiated by the break of head-to-head links or the weak bonds at the chain end [31, 32], these weak links may be reduced while the St units were introduced to the chains of PMMA, hence inhibiting the degradation at lower temperatures. As a result, the heat resistance of P(MMA-co-St) was significantly improved compared to pure-PMMA.
Figure 10. TGA curves (a) and DTG curves (b) of P(MMA-co-St).
Download figure:
Standard image High-resolution imageTable 4. Tonset and Td50 of P(MMA-co-St).
| Parameters | PMMA | P(MMA-co-St)-5 | P(MMA-co-St)-10 | P(MMA-co-St)-20 |
|---|---|---|---|---|
| Tonset (℃) | 253 | 262 | 279 | 290 |
| Td50 (℃) | 336 | 352 | 361 | 368 |
Mechanical properties of P(MMA-co-St)
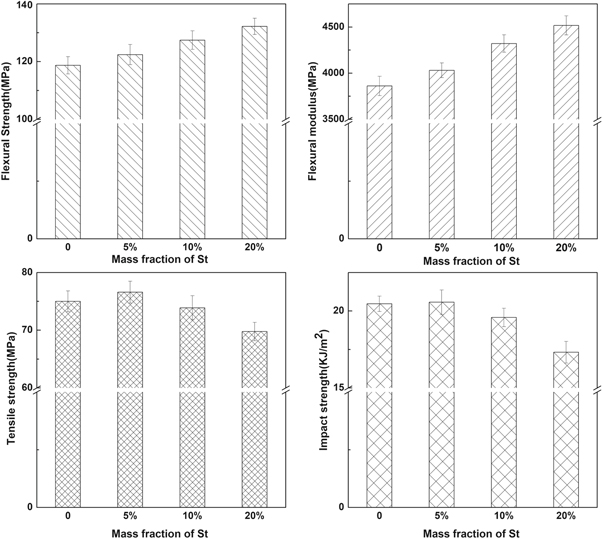
The mechanical performance of P(MMA-co-St) is presented in figure 11. It can be seen that when the ratio of St was 5 wt% or 10 wt%, the tensile strength and impact strength of P(MMA-co-St) weren't significantly declined compared to PMMA-C, which may be due to the higher molecular weight of P(MMA-co-St). Therefore, although the introduction of St units would partially weaken the force between the molecular chains, the mechanical properties wouldn't significantly decrease due to the increase of molecular weight. Only when the ratio of St reached to 20 wt%, the tensile strength and impact strength showed the obvious decline. On the other hand, the flexural strengths and flexural modulus of P(MMA-co-St) all increased with the content of St. The flexural strength and flexural modulus of P(MMA-co-St)-10 increased by 7% and 12% compared with PMMA-C, respectively. These results indicated that the rigidity of molecular chains was enhanced by introducing the St units, making the copolymers possess better bending resistance.
Figure 11. Mechanical properties of P(MMA-co-St).
Download figure:
Standard image High-resolution imageConclusions
The occurrence of Trommsdorff effect could be regulated by changing the initial viscosity of polymerization solution, and the bulk polymerization of MMA would be in the Trommsdorff effect region while the initial viscosity exceeded 1000 ± 100 Pa · s. Therefore, 1000 ± 100 Pa · s of viscosity could be a criterion for the occurrence of Trommsdorff effect. Then the continuous bulk copolymerization of MMA and St was completed in a new inverted two-stage extruder, with the 'blocking unit' introduced to improve the residence time of copolymerization system. The copolymerization rate was greatly accelerated and the copolymers with higher molecular weight and narrow distribution were obtained by effectively utilizing the Trommsdorff effect, the conversion were above 98%. The introduction of St units could significantly reduce the melt viscosity of PMMA. When the St content was 10 wt%, the melt flow rate was increased by 3 times. The Tonset and Td50 of P(MMA-co-St)-10 were increased by 26 ℃ and 25 ℃ compared with pure-PMMA, respectively, and the copolymer still possessed good mechanical properties. The results in this article provide important reference and guidance for the industrialization of MMA-St copolymer prepared by reactive extrusion.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 51373052, No. 51573043).