- 1Institute of Health Innovations and Outcomes Research, Feinstein Institutes for Medical Research, Manhasset, NY, United States
- 2Duke Surgery, Duke University Medical Center, Durham, NC, United States
Vascular composite allotransplantation (VCA) is a field under research and has emerged as an alternative option for the repair of severe disfiguring defects that result from infections or traumatic amputation in a selected group of patients. VCA is performed in centers with appropriate expertise, experience and adequate resources to effectively manage the complexity and complications of this treatment. Lifelong immunosuppressive therapy, immunosuppression associated complications, and the effects of the host immune response in the graft are major concerns in VCA. VCA is considered a quality of life transplant and the risk-benefit ratio is dissimilar to life saving transplants. Belatacept seems a promising drug that prolongs patient and graft survival in kidney transplantation and it could also be an alternative approach to VCA immunosuppression. In this review, we are summarizing current literature about the role of costimulation blockade, with a focus on belatacept in VCA.
Introduction
Vascular composite allotransplantation (VCA) is a field under research and has emerged as an alternative option for the repair of severe disfiguring defects that result from infections or traumatic amputation in a selected group of patients (1). VCA consist of anatomically distinct tissues such as skin, muscles, connective tissue stroma, bones and neurovascular elements that are transplanted as functional complexes (2–5). It can be applied to various body parts, such as the face, upper or lower extremity, the larynx, the abdominal wall, as well as intra-abdominal organs, such as the spleen, the adrenals or genitourinary organs (5–7).
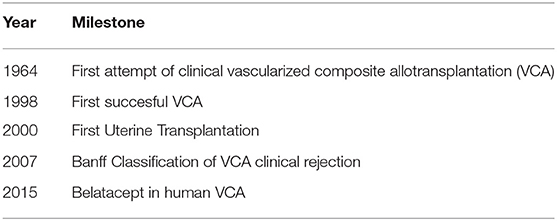
The first attempt for a human hand transplantation was done in 1964 in Ecuador, but acute rejection (AR) led to graft amputation at 3 weeks postoperatively, despite the use of combined immunosuppression therapy with azathioprine and prednisone (8) (Table 1). Many years have passed since then, until another VCA transplantation was performed. Newer immunosuppressive drugs emergenced, such as cyclosporine and tacrolimus, combined with improved solid organ transplantation (SOT) and non-human primates (NHP) VCA outcomes have led to a new era in human VCA transplantation (9–15). The first technically successful forearm transplantation was performed in 1998 in Lyon, France, but the initially viable graft was eventually rejected and amputated due to patient non-compliance with immunosuppressive treatment, consisting of tacrolimus, mycophenolic acid and prednisone (16, 17). Hand transplantation in the United States was firstly performed in Louisville, Kentucky in 1999 with functional improvement compared to prosthesis and allograft currently surviving 20 years post-transplantation (9, 18). Hand transplantation is currently the most common VCA performed clinically.
In 2005, the first face transplantation was reported by the Amiens transplantation team in a woman suffering from traumatic amputation of the lower face, including distal nose, lips, chin, and lateral face parts (19). Since then at least 46 facial transplantations have been performed worldwide in patients with burn injuries, animal bites or malignancies (20–23). Face transplantation re-establishes the ability of patients to speak, feed themselves and express their emotional status, thus facilitating an adequate social life (6). Immunosuppression regimens are similar to the scheme used in hand transplantations, consisting of induction therapy, such as anti-thymocyte globulin (ATG), alemtuzumab or basiliximab followed by the standard triple drug regimen, which includes tacrolimus, mycophenolate mofetil (MMF) and steroids (6, 24–26).
Advances in the field of VCA led to the first time uterine transplantation (UTx). It was first attempted by Fageeh et al. in Saudi Arabia in 2000 on a 26 year-old female in the context of previously performed hysterectomy due to post-partum hemorrhage. The uterus graft underwent acute thrombosis 3 months post-transplantation and eventually hysterectomy was performed (27). The first successful pregnancy and livebirth after UTx was achieved in Gothenburg, Sweden in 2015, demonstrating that UTx may be a feasible fertility-restoring option for women with uterine factor infertility (28). The Swedish group has paved the way in the establishment of UTx as a viable option for infertility and are leaders in uterus tissue engineering (29) and minimally invasive UTx (30, 31). Ejzenberg et al. recently reported the first successful livebirth from deceased donor in Brazil in a young patient with Mayer-Rokitansky-Küster-Hauser syndrome (32). In the United States, the field is rapidly growing. In 2017, the first 5 cases of UTx from living donors were reported (33). In 2018, the same group reported the first livebirth after UTx from altruistic living donor (34). To date, more than 60 UTx have been performed globally and 18 offspring have been reported to have been successfully delivered (35).
The main limitation of currently used immunosuppression regimens are side effects. Specifically, tacrolimus, which is a calcineurin inhibitor (CNI) is a core component of maintenance regimens in VCA worldwide and has been reported as one of the main causes of complications in VCA. Barth et al. reviewed the VCA experience and identified 4 cases (3 limb and 1 face) with renal failure or progressive renal dysfunction up to 8 years post-transplantation. One patient has been transplanted and 3 are listed for renal transplantation (36). Alternative agents have emerged in an effort to minimize or replace the use of tacrolimus and associated side effects. Belatacept-based immunosuppression in preclinical VCA models and clinical VCA has recently emerged as a promising alternative treatment to counteract long-term adverse effects of currently used chronic immunosuppression agents (25, 37–39). Belatacept is a fusion protein (CTLA4-IgG1) that targets the CD28/B7 costimulation between T- and B-lymphocytes. The BENEFIT phase 3 randomized controlled study revealed increased patient and graft survival as well as improved renal function with belatacept at seven years post-transplantation compared to cyclosporine-based regimen (40). Furthermore, the risk of death or long-term graft loss reduced by 43% with both belatacept regimens tested and the glomerular filtration rate (GFR) increased compared to cyclosporine-nephrotoxicity GFR decrease (40). In this review, we are focusing on summarizing current literature about the role of costimulation blockade in VCA.
VCA and Rejection
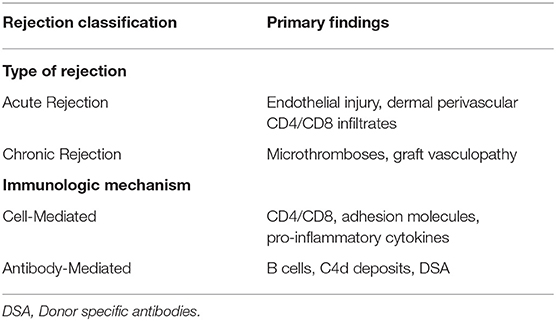
The increasing numbers of VCA along with advances in immunomodulation schemes mandate the need of a universally accepted histological classification. The Banff 2007 classification was a milestone in the characterization and appropriate reporting of VCA rejection. The skin as a visible component of the transplant provides an easily and accessible monitored graft area facilitating AR recognition. According to Banff 2007 the skin rejection severity is classified in five grades ranging between 0 and IV (41). AR manifests with skin lesions, such as macules, papules, erythema, edema and nail changes (42, 43). AR initially involves neutrophils and T-cells producing chemoattractive factors acting on macrophages (IFN-γ) (Table 2). If not reversed, AR progresses from mild perivascular dermis inflammation to epidermal and adnexal cellular infiltration and eventually irreversible epidermal necrosis (41, 44). Repeated episodes of AR are considered important contributors to chronic allograft dysfunction. Multiple AR episodes result in persistent chemokine elevation and macrophage graft infiltration, resulting in fibroblast proliferation and collagen deposition through macrophage secreted cytokines (FGFβ, TGFβ and PDGF) (45, 46). Histopathology of chronic graft functional deterioration involves myointimal proliferation, fibrosis, vasculopathy and parenchymal structural dysregulation (45, 47). Despite initial beliefs underestimating the role of antibodies in VCA graft damage, it is currently established that antibody-mediated rejection is also an important process affecting graft viability (48, 49). The vascular component of the graft is a target in chronic rejection and by donor specific antibodies (DSA), which may develop years after transplantation and have been associated with CNI immunosuppression sparing regimens (50, 51).
VCA Immunosupression: Conventional Immunosuppressive Drugs
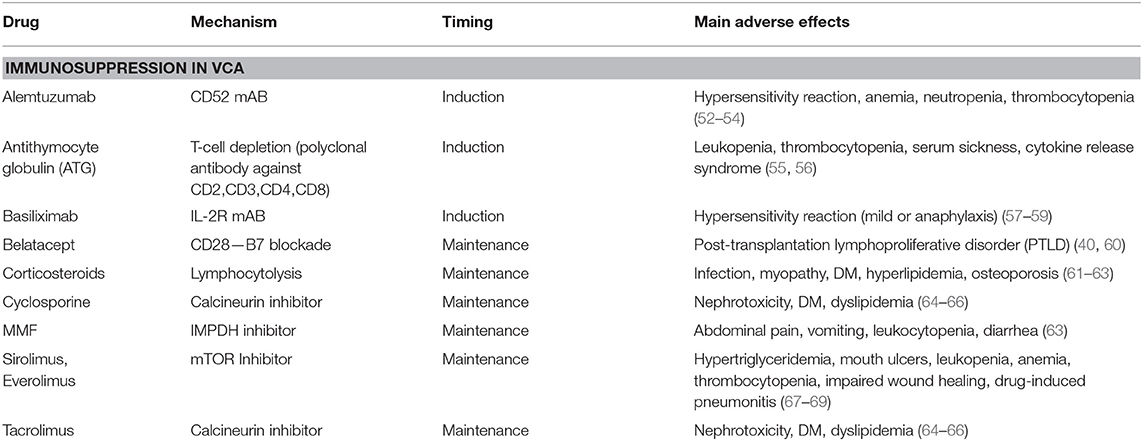
Currently used immunosuppressive drug regimens protect from early graft loss but are unable to prevent rejection in up to 90% of all VCA recipients and are associated with serious adverse effects (6) (Table 3). Opportunistic infections due to viruses, such as EBV, CMV, HSV-1, have been extensively reported and investigated in SOT recipients and have been identified as major complications affecting VCA patients (6, 70–72). Cancer commonly affects transplant recipients and is an emerging issue in VCA transplantation, in the form of de novo tumorigenesis, associated with immunosuppression per se or viral reactivation (EBV related lymphomas), or tumor recurrence (20, 73). The most commonly used induction agent in VCA is antithymocytic globulin (ATG) and acts through T-cell depletion as a polyclonal antibody directed against the CD2, CD3, CD4, and CD8 molecules. ATG induction results in decreased T-cell mediated rejection, which is an common observation in VCA rejection (74–76). ATG side effects include leukopenia, thrombocytopenia, serum sickness, cytokine release syndrome, and infections (55, 56). Corticosteroids are considered as a milestone of transplantation immunosuppressive therapy. Nevertheless, their side-effects, such as myopathy, diabetes mellitus, hyperlipidemia, osteoporotic fractures, impaired wound healing, have led to the emergence of steroid sparing regimens with promising results in SOT (61–63). Tacrolimus and cyclosporine are calcineurin inhibitors and their well-known detrimental effects include impaired kidney function (acute and chronic nephrotoxicity), glucose metabolism (hyperglycemia) and lipid metabolism (dyslipidemia) (64–66). Tacrolimus to sirolimus (mTOR kinase inhibitor) conversion has been successfully used in VCA in order to counteract renal toxicity (77). Mycophenolate Mofetil (MMF), commonly used as maintenance drug, acts as inosine monophosphate dehydrogenase (IMPDH) inhibitor and interferes with de novo purine nucleotide synthesis, which is essential for the proliferation of lymphocytes (78). Main adverse reactions associated with MMF include abdominal pain, vomiting, leukocytopenia and diarrhea (63).
VCA Immunosuppresion: Monoclonal Antibodies
The optimal immunosuppressive regimen would prevent rejection as well as have minimal or no major toxicity over a prolonged period of use. Monoclonal antibodies (mAbs) have been tested as promising agents in VCA immunosuppressive regimens.
Alemtuzumab and Basiliximab
Alemtuzumab, a humanized mAb targeting CD52 (GPI-linked surface protein of mature lymphocytes) that causes B and T lymphocyte depletion, has been used in the prevention of SOT AR as well as in the treatment of VCA AR episodes (24, 79, 80). Basiliximab, a chimeric mAb specifically binding to interleukin-2 receptor, has been tested with promising results in SOT recipients and has also been used as induction therapy in VCA recipients (26, 81–83).
Belatacept: Mechanism, Benefits and Side Effects
The development of belatacept along with its prototype molecule CTLA4-Ig introduced a new class of immunosuppressive agents called costimulation blockade agents (84). T-cells become activated in the presence of three signals: One signal is mediated through the interaction of T-cell receptor (TCR) and major histocompatibility complex (MHC) molecules, an additional accessory costimulatory signal is mediated through the interaction of other cell surface molecules and the third signal is delivered in the form of cytokines (85). Naïve helper (CD4+) and cytotoxic (CD8+) T-cells must undergo activation in order to become effectors cells able to participate in graft rejection (86). Belatacept is a fusion protein consisting of cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4 or CD152) and IgG1 Fc portion acting through the blockade of CD28 (T-cell surface) and B7 (B-cell surface) costimulation. Belatacept was approved as a CNI replacement therapy in kidney transplant recipients with significantly better renal function, patient and graft survival compared to cyclosporine (40, 87). However, the BENEFIT study demonstrated high cumulative rates of acute rejection in both high (24.4%) and low (18.3%) intensity belatacept groups compared to cyclosporine (11.4%) at 7 years post-transplantation. Higher rate of donor specific DSA formation was observed in cyclosporine treated patients (17.8%) compared to the high intensity (1.9%) and low intensity (4.6%) belatacept regimens (40). Belatacept is more effective in the prevention of the development of DSA, but may be inferior to CNIs in the short term in the prevention of acute cellular rejection. Safety profile is comparable, if not better, to CNI drugs (88). Specifically, belatacept offers better blood pressure management, lipid metabolic profile and lower incidence of post-transplantation diabetes (89, 90). In addition, there is no difference in risk of developing cancer or infectious complications in kidney transplantation between belatacept and CNI (90). Belatacept treatment is limited to EBV-seropositive recipients, because some seronegative patients of the BENEFIT study, who were given higher doses, developed post-transplantation lymphoproliferative disorder (PTLD) (40, 60). In addition, belatacept is not indicated in liver transplantation based on a phase II study report that associated belatacept with increased graft loss and mortality compared to MMF + tacrolimus (91).
CTLA4-Ig and Belatacept in VCA Experimental Models
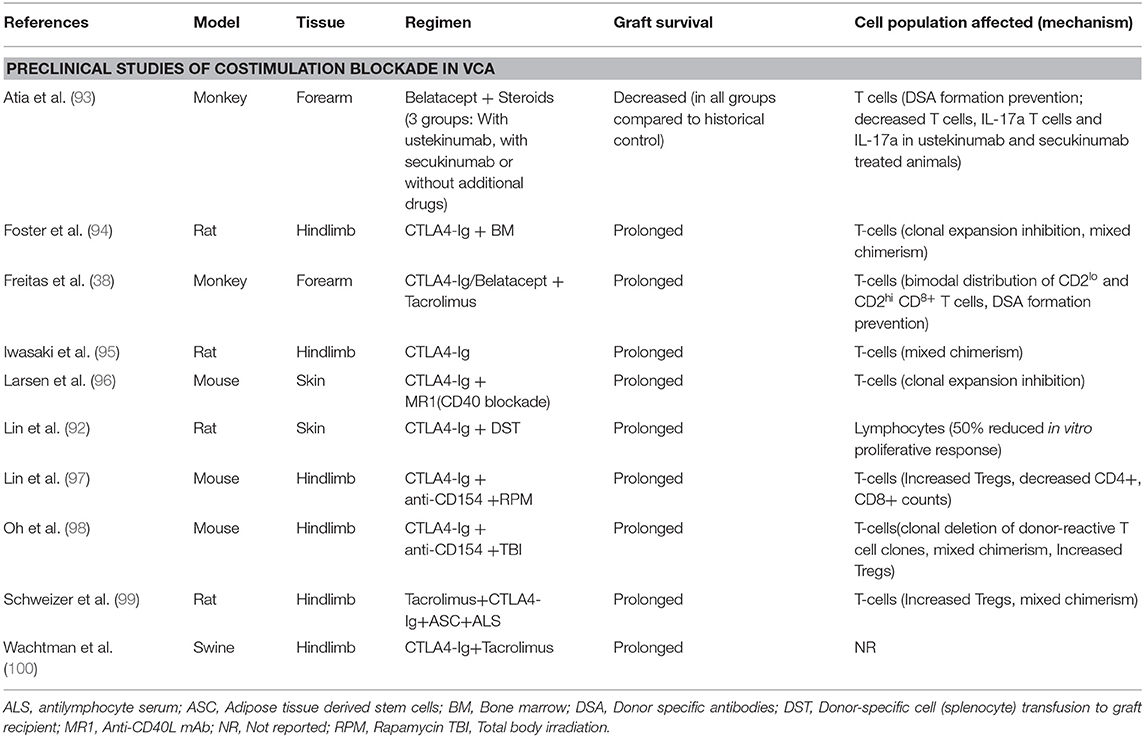
Costimulation immunosuppression with CTLA4-Ig has been shown to prolong survival of donor skin grafts in rat cardiac allograft transplantation compared to third-party skin grafts, indicating donor-specific tolerance induction (92) (Table 4). Combination of CD28 and CD40 pathway blockade promotes survival of allogeneic skin grafts in the context of T-cell clonal expansion prevention (96). Iwasaki et al. used a rat hind limb VCA model to demonstrate that CTLA4-Ig regimen on day 2 significantly prolongs graft survival compared to vehicle and control groups (hIgG administration and no treatment, respectively) (Median graft survival: 20.5, 9, and 9 days, respectively, p < 0.01) and all CTLA4-Ig treated histologic specimens remained unaffected at 7 days post-transplantation (95). In addition, the same study showed that CTLA4-Ig optimally inhibits allograft rejection when administered on postoperative days 1 or 2 compared to immediate post-transplant treatment (95). Foster et al., using a model consisting of fully mismatched donor and recipient rats, showed that donor bone marrow (BM) administered to recipients, at 4 weeks prior to hind limb VCA transplantation, combined with CTLA4-Ig could effectively prevent acute and chronic rejection of the allograft (94). VCA hind limb allograft survival in swines has been shown to benefit significantly by CTLA4-Ig + Tacrolimus combination compared to Tacrolimus + BM transplantation + Irradiation or Tacrolimus only regimens, with a great impact on skin component rejection prevention (100). Lin et al. utilized a combination of anti-CD154 (anti-CD40L), CTLA4-Ig and rapamycin (RPM) in mice osteomyocutaneous allografts transplantation and reported long-term survival in the anti-CD154 + CTLA4-Ig+RPM group compared to anti-CD154 + CTLA4-Ig or RPM only groups (Median survival time: 103, 33, 45.8 days, respectively) (97). In the aforementioned study, long graft survival was associated with increased number of T-regulatory cells (Tregs) and decreased CD4+ and CD8+ counts (97). More recently, Oh and colleagues tested the combination of CTLA4-Ig + anti-CD154 + total body irradiation in a fully MHC-mismatched mouse hindlimb model and reported a graft survival of over seven months compared to 82 days in the group treated with CTLA4-Ig + anti-CD154 only (98). Lastly, Schweizer et al. used adipose-derived mesenchymal stem cells combined with CTLA4-Ig and antilymphocyte serum in a rat hindlimb model, in addition to tacrolimus, and achieved an over 4 months rejection free allograft survival compared to control groups (median graft survival <35 days) (99).
Preclinical data in VCA have already demonstrated the efficacy of belatacept as a maintenance treatment in VCA (38, 39). Freitas et al. reported that, in a cynomolgus monkey model of forearm VCA, costimulation blockade in the form of CTLA4-Ig or belatacept combined with tacrolimus improved graft survival and prevented DSA formation compared to tacrolimus + steroids (38). Recently, Atia et al. investigated the effect of belatacept in combination with Th17 response inhibitory drugs (ustekinumab and secukinumab) in a rhesus macacques model of VCA. The comparison with a historic cohort, treated with the standard immunosuppression (Tacrolimus, MMF, Methylprednisolone), revealed significantly shorter interval to acute rejection in all groups (≤14 days), independent of the Th17 inhibition, compared to controls (mean survival = 31.1 days). However, historic controls without costimulation blockade showed a significant increase in DSA production at rejection, while all belatacept treated animals did not develop post-transplant DSA at rejection (93).
Belatacept in Clinical VCA
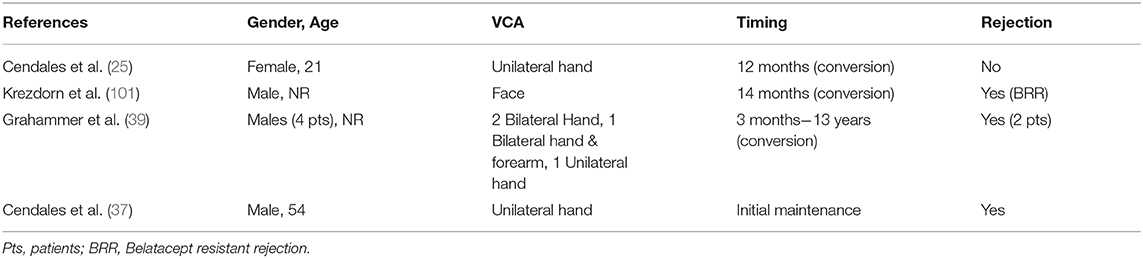
Belatacept has been successfully used as CNI replacement treatment to counteract CNI-induced nephrotoxicity in a 21 year old female hand transplant recipient with left wrist amputation due to Kawasaki vasculitis affecting the extremities. The patient developed recurrent episodes of acute rejection with alloantibody formation and initial maintenance treatment consisting of tacrolimus, MMF and steroids was replaced by belatacept, sirolimus and steroids at 12 months post-transplantation (25) (Table 5). Belatacept based costimulation blockade was applied based on the diagnostic confirmation of antibody-mediated rejection and previous results of belatacept efficacy on the prevention of alloantibody formation (25, 102). After conversion, at 42 months post-transplantation and 30 months on belatacept, no episodes or signs of rejection occurred in the normally functioning allograft (25).
Krezdorn et al. investigated the immunologic response of a face transplantation recipient who developed belatacept resistant rejection (BRR) after belatacept conversion in the context of tacrolimus and rapamycin adverse effects (101). Initially, tacrolimus + MMF + prednisone were applied with tacrolimus to rapamycin conversion at 11 months post-transplantation due to impaired renal function and neurotoxicity as well as CMV infection. Subsequently, at 14 months post-transplantation, further deterioration of renal function and cell-mediated rejection led to belatacept conversion. Nevertheless, 4 months post-conversion the patient developed rejection and was additionally treated with low-dose tacrolimus to achieve remission (101). Previous data derived from costimulation-based regimens in kidney transplantation revealed that a specific subset of CD4+ T cells (CD4+CD57+PD1-) with cytolytic properties may act as efficient high-risk marker of BRR (103). Nevertheless, this CD4+ subset was not significantly elevated in this face transplant recipient prior to or after belatacept initiation. Interestingly, both Tregs and Tfh cell counts were decreased during belatacept treatment, whereas Th1 and Th17 counts increased (101). Belatacept-induced Tfh count decrease has been previously shown to suppress humoral immunity and antibody-mediated rejection (104).
Recently, belatacept was tested as maintenance treatment in 4 male hand-transplanted patients with beneficial results and limitations as well. In two DSA-negative bilateral hand recipients, belatacept treatment was not associated with rejection in spite of decreases in tacrolimus dosage (patient 1) or cessation of everolimus administration (patient 2) (39). Patient 3 received bilateral forearm transplantation and had suffered from recurrent cell-mediated rejection and one DSA (+) rejection episode prior to belatacept initiation at 9 years post-transplantation. He remained free of rejection without detection of DSA along with improved graft macroscopic image and function. The last patient received belatacept at 6 years post-transplantation due to CNI caused nephrotoxicity, but at 2 months of costimulation-blockade acute rejection occurred and was treated with alemtuzumab conversion. Eventually, 8 months later resistant rejection led to removal of the transplanted hand. Patient 4 immunologic profile revealed that CD4+CD57+ T-cells were increased compared to long-term graft survival patients (39).
Our group investigated the role of de-novo belatacept in VCA (37). A 54 year-old male transplant recipient, suffering from traumatic amputation of the left hand, was treated with belatacept, MMF, steroids and tacrolimus, followed by conversion to sirolimus at 6 months. At 8 months post-transplantation macroscopically (erythematous maculopapular rash) and microscopically confirmed rejection Banff III (41), which was successfully treated with IV steroids. At 20 months post-transplantation the patient was reported to be free of rejection, with improved graft function in daily activities and maintained on belatacept + MMF + prednisone (37). This study demonstrated that belatacept can be incorporated as a core component of antirejection regimens, minimizing the use of CNI and their long-term adverse effects.
Belatacept in VCA: Advantages and Limitations
Currently, belatacept seems as a promising agent that prolongs the rejection free survival when added to tacrolimus in experimental VCA models (38). However, belatacept in combination with steroids alone failed to prevent acute rejection and resulted in an average rejection free survival (time from transplant to early signs of rejection) of 10 days compared to an average rejection free survival 31.1 days in animals treated with tacrolimus, MMF and steroids (93, 105). As it has been shown by us and others, the use of belatacept resulted in inhibition of DSA formation (39, 93). As anticipated and similar to other organ transplants graft vasculopathy and antibody mediated rejection in VCA are associated with the presence of DSA or C4d deposits (106–110). The incidence of acute rejection in VCA has been reported to 85% within the first year (39, 76), which is higher than other solid organ transplants (111–113). Belatacept is associated with a higher incidence of acute rejection episodes in kidney (40). Considering that VCA has a higher reported incidence of skin changes attributed to rejection, even in MHC-matched VCA transplants (100), this would be an area to study and to report as more cases are performed. An important consideration is differential diagnosis. Specifically, in VCA, the skin is the monitoring tool for rejection in VCA and studies by our VCA collaborative initiative have shown that skin is the harbinger of rejection (114). However, the skin changes in VCA -although characteristic- they are non- specific (115). Similarly, they are not limited to alloimmune injury. Thus, the diagnosis of rejection can be challenging as multiple unrelated inflammatory dermatoses can mimic alloimmune driven acute rejection (e.g., infectious, drug toxicity) (115). As the field continues to develop and more data become available particularly as they relate to differential diagnosis of rejection, the incidence of the skin changes attributed to rejection may change.
Based on studies in swine, CTLA4-Ig and CNIs are effective in preventing allograft rejection. In a study by Wachtman et al. in swine CTLA4-Ig was utilized in combination with tacrolimus (CNI was stopped at 30 days). The CTLA4-Ig regimen resulted in a prolonged survival in animals (indefinite in two animals) while the tacrolimus alone group resulted in rejection 2 days after tacrolimus cessation (100). Our studies on NHPs show the benefit of belatacept in VCA. The addition of de novo belatacept to a regimen consisting of tacrolimus, steroids, and conversion to sirolimus, significantly prolonged the rejection free survival (Up to 140 days in belatacept vs. 14 days in non-belatacept regimen) (38). Based on these findings in large animal models and our human clinical trial (NCT02310867), a regimen that deserves ongoing consideration is de novo belatacept, calcineurin inhibitors for the initial 6 months to avoid the negative impact of sirolimus on wound healing (38) followed by conversion to sirolimus (25).
A main clinical concern with belatacept treatment is the risk of post-transplantation lymphoproliferative disorder (PTLD) in EBV seronegative transplant recipients (40, 116). In addition and similar to other organ transplants, immunomodulation during conversion requires surveillance for potential increase rejection. As described by Grahammer et al., hand transplant recipients who remained clinically stable prior to and after belatacept conversion, were free of cellular rejection and DSA formation at time of belatacept initiation (39).
Costimulation blockade alone is ineffective in inducing long-term graft allograft survival or tolerance in animal transplant models (98, 117, 118). The inhibition of CD28/B7 costimulation pathway affects various immune cells, including Tregs and Th17 cells, while the time course and regimen intensity are critical predictors of the ultimate response (119). Costimulation pathway CD28/B7 is important for the activation of Tregs, which is important for the induction of tolerance (119, 120). An unfavorable effect of belatacept on Tregs was shown in a face transplant recipient who presented with rejection 4 months after conversion to belatacept (101). It has also been shown that costimulation blockade has a is limited effect on memory T cells, which are less dependent on CD28/B7 activation and have been implicated in belatacept resistant rejection (39, 121–125). Potential co-targeting of these memory cells to bypass this limitation may predispose to increase risk of infections (119). In addition, costimulation blockade with agents targeting the B7 molecule, not only inhibit CD28/B7 interaction, but prevent CTLA4/B7 interaction and programmed death-ligand 1 (PD-L1)/B7 interaction and T-cell co-inhibition. Impairment of T-cell co-inhibition results in ineffective control of alloreactive T-cell activation, including effector memory T cells and Th17 cells (119, 126, 127).
Summary
VCA is a field under development and is performed in centers with appropriate expertise, experience and adequate resources to effectively manage the complexity and complications of this treatment option. Similar to other solid organ transplants, lifelong immunosuppressive therapy, their complications, and the effects of the alloimmune response in the graft are major concerns in VCA. VCA is a quality of life transplant and the risk-benefit ratio is dissimilar to life saving transplants. Belatacept seems a promising drug that prolongs patient and graft survival in solid organ transplantation and it could also be an alternative approach to VCA immunosuppression. A regimen that deserves ongoing consideration is de novo belatacept to avoid chronic CNI exposure.
Author Contributions
DM and LC: conception or design of the work. DG and DM: data collection. DG, DM, and LC: data analysis and interpretation, drafting the article, critical revision of the article, and final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gorantla VS, Plock JA, Davis MR. Reconstructive transplantation: evolution, experience, ethics, and emerging concepts. In: Subramaniam K, Sakai T, editors. Anesthesia and Perioperative Care for Organ Transplantation. New York, NY: Springer (2017) p. 539–52.
2. Diaz-Siso JR, Fischer S, Sisk GC, Bueno E, Kueckelhaus M, Talbot S, et al. Initial experience of dual maintenance immunosuppression with steroid withdrawal in vascular composite tissue allotransplantation: steroid withdrawal in VCA. Am J Transplant. (2015) 15:1421–31. doi: 10.1111/ajt.13103
3. Murphy BD, Zuker RM, Borschel GH. Vascularized composite allotransplantation: an update on medical and surgical progress and remaining challenges. J Plast Reconstr Aesthetic Surg. (2013) 66:1449–55. doi: 10.1016/j.bjps.2013.06.037
4. Vernon R, Wang J, Song M, Wilson N, Moris D, Cendales L. Vascularized composite allotransplantation: a functional hind limb model in mice. J Surg Res. (2020) 250:119–24. doi: 10.1016/j.jss.2019.12.042
5. Cherikh WS, Cendales LC, Wholley CL, Wainright J, Gorantla VS, Klassen DK, et al. Vascularized composite allotransplantation in the United States: a descriptive analysis of the organ procurement and transplantation network data. Am J Transplant. (2019) 19:865–75. doi: 10.1111/ajt.15062
6. Schneeberger S, Landin L, Jableki J, Butler P, Hoehnke C, Brandacher G, et al. Achievements and challenges in composite tissue allotransplantation: achievements and challenges in reconstructive transplantation. Transpl Int. (2011) 24:760–9. doi: 10.1111/j.1432-2277.2011.01261.x
7. Wang J, Wu J, Moris D, Hayes B, Abraham SN, Cendales LC. Introducing a novel experimental model of bladder transplantation in mice. Am J Transplant. (2020) doi: 10.1111/ajt.15912. [Epub ahead of print].
9. Jones JW, Ustüner ET, Zdichavsky M, Edelstein J, Ren X, Maldonado C, et al. Long-term survival of an extremity composite tissue allograft with FK506-mycophenolate mofetil therapy. Surgery. (1999) 126:384–8. doi: 10.1016/S0039-6060(99)70181-9
10. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. (2000) 342:605–12. doi: 10.1056/NEJM200003023420901
11. Rosenthal JT, Hakala TR, Iwatsuki S, Shaw BW, Starzl TE. Cadaveric renal transplantation under cyclosporine-steroid therapy. Surg Gynecol Obstet. (1983) 157:309–15.
12. Salaman JR. Renal transplantation without steroids. Pediatr Nephrol Berl Ger. (1991) 5:105–7. doi: 10.1007/BF00852865
13. Ochiai T, Nakajima K, Nagata M, Suzuki T, Asano T, Uematsu T, et al. Effect of a new immunosuppressive agent, FK 506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. (1987) 19(Pt. 2):1284–6.
14. Todo S, Podesta L, ChapChap P, Kahn D, Pan C-E, Ueda Y, et al. Orthotopic liver transplantation in dogs receiving FK-506. Transplant Proc. (1987) 19(Suppl. 6):64–7.
15. Fung JJ, Starzl TE. FK506 in solid organ transplantation. Ther Drug Monit. (1995) 17:592–5. doi: 10.1097/00007691-199512000-00008
16. Dubernard JM, Owen E, Herzberg G, Lanzetta M, Martin X, Kapila H, et al. Human hand allograft: report on first 6 months. Lancet Lond Engl. (1999) 353:1315–20. doi: 10.1016/S0140-6736(99)02062-0
17. Dubernard JM, Owen E, Lefrançois N, Petruzzo P, Martin X, Dawahra M, et al. First human hand transplantation. Transpl Int. (2000) 13 (Suppl. 1):S521–4. doi: 10.1111/j.1432-2277.2000.tb02095.x
18. Kaufman CL, Bhutiani N, Ramirez A, Tien HY, Palazzo MD, Galvis E, et al. Current status of vascularized composite allotransplantation. Am Surg. (2019) 85:631–7. doi: 10.1177/000313481908500628
19. Devauchelle B, Badet L, Lengelé B, Morelon E, Testelin S, Michallet M, et al. First human face allograft: early report. Lancet Lond Engl. (2006) 368:203–9. doi: 10.1016/S0140-6736(06)68935-6
20. Giatsidis G, Sinha I, Pomahac B. Reflections on a decade of face transplantation. Ann Surg. (2017) 265:841–6. doi: 10.1097/SLA.0000000000001760
21. Rifkin WJ, David JA, Plana NM, Kantar RS, Diaz-Siso JR, Gelb BE, et al. Achievements and challenges in facial transplantation. Ann Surg. (2018) 268:260–70. doi: 10.1097/SLA.0000000000002723
22. Iske J, Nian Y, Maenosono R, Maurer M, Sauer IM, Tullius SG. Composite tissue allotransplantation: opportunities and challenges. Cell Mol Immunol. (2019) 16:343–9. doi: 10.1038/s41423-019-0215-3
23. Alberti FB. Face transplants as surgical acts and psychosocial processes. Lancet. (2020) 395:1106–7. doi: 10.1016/S0140-6736(20)30684-X
24. Kanitakis J, Petruzzo P, Badet L, Gazarian A, Thaunat O, Testelin S, et al. Chronic rejection in human vascularized composite allotransplantation (hand and face recipients): an update. Transplantation. (2016) 100:2053–61. doi: 10.1097/TP.0000000000001248
25. Cendales L, Bray R, Gebel H, Brewster L, Elbein R, Farthing D, et al. Tacrolimus to belatacept conversion following hand transplantation: a case report: belatacept in hand transplantation. Am J Transplant. (2015) 15:2250–5. doi: 10.1111/ajt.13217
26. Petruzzo P, Lanzetta M, Dubernard J-M, Landin L, Cavadas P, Margreiter R, et al. The International registry on hand and composite tissue transplantation. Transplantation. (2010) 90:1590–4. doi: 10.1097/TP.0b013e3181ff1472
27. Fageeh W, Raffa H, Jabbad H, Marzouki A. Transplantation of the human uterus. Int J Gynecol Obstet. (2002) 76:245–51. doi: 10.1016/S0020-7292(01)00597-5
28. Brännström M, Johannesson L, Bokström H, Kvarnström N, Mölne J, Dahm-Kähler P, et al. Livebirth after uterus transplantation. Lancet Lond Engl. (2015) 385:607–16. doi: 10.1016/S0140-6736(14)61728-1
29. Tiemann TT, Padma AM, Sehic E, Bäckdahl H, Oltean M, Song MJ, et al. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol Hum Reprod. (2020) 26:167–78. doi: 10.1093/molehr/gaaa009
30. Ayoubi JM, Carbonnel M, Pirtea P, Kvarnström N, Brännström M, Dahm-Kähler P. Laparotomy or minimal invasive surgery in uterus transplantation: a comparison. Fertil Steril. (2019) 112:11–8. doi: 10.1016/j.fertnstert.2019.05.038
31. Carbonnel M, Dahm-Kähler P, Revaux A, Brännström M, Ayoubi J-M. Adapting surgical skills from robotic-assisted radical hysterectomy in cervical cancer to uterine transplantation: a look to an optimistic future! J Robot Surg. (2020). doi: 10.1007/s11701-020-01058-7. [Epub ahead of print].
32. Ejzenberg D, Andraus W, Baratelli Carelli Mendes LR, Ducatti L, Song A, Tanigawa R, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet Lond Engl. (2019) 392:2697–704. doi: 10.1016/S0140-6736(18)31766-5
33. Testa G, Koon EC, Johannesson L, McKenna GJ, Anthony T, Klintmalm GB, et al. Living donor uterus transplantation: a single center's observations and lessons learned from early setbacks to technical success. Am J Transplant. (2017) 17:2901–10. doi: 10.1111/ajt.14326
34. Testa G, McKenna GJ, Gunby RT, Anthony T, Koon EC, Warren AM, et al. First live birth after uterus transplantation in the United States. Am J Transplant. (2018) 18:1270–4. doi: 10.1111/ajt.14737
35. Daolio J, Palomba S, Paganelli S, Falbo A, Aguzzoli L. Uterine transplantation and IVF for congenital or acquired uterine factor infertility: a systematic review of safety and efficacy outcomes in the first 52 recipients. PLoS ONE. (2020) 15:e0232323. doi: 10.1371/journal.pone.0232323
36. Barth RN, Shores JT, Brandacher G, Levine MH, Weissenbacher A, Nam AJ, et al. Renal Failure as a Complication of Vascularized Composite Allotransplantation. ATC Abstracts. Available online at: https://atcmeetingabstracts.com/abstract/renal-failure-as-a-complication-of-vascularized-composite-allotransplantation/ (accessed May 10, 2020).
37. Cendales LC, Ruch DS, Cardones AR, Potter G, Dooley J, Dore D, et al. De novo belatacept in clinical vascularized composite allotransplantation. Am J Transplant. (2018) 18:1804–9. doi: 10.1111/ajt.14910
38. Freitas AM, Samy KP, Farris AB, Leopardi FV, Song M, Stempora L, et al. Studies introducing costimulation blockade for vascularized composite allografts in nonhuman primates: costimulation blockade in VCA. Am J Transplant. (2015) 15:2240–9. doi: 10.1111/ajt.13379
39. Grahammer J, Weissenbacher A, Zelger BG, Zelger B, Boesmueller C, Ninkovic M, et al. Benefits and limitations of belatacept in 4 hand-transplanted patients. Am J Transplant. (2017) 17:3228–35. doi: 10.1111/ajt.14440
40. Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. (2016) 374:333–43. doi: 10.1056/NEJMoa1506027
41. Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology: Banff CTA allograft pathology classification. Am J Transplant. (2008) 8:1396–400. doi: 10.1111/j.1600-6143.2008.02243.x
42. Schneeberger S, Ninkovic M, Piza-Katzer H, Gabl M, Hussl H, Rieger M, et al. Status 5 years after bilateral hand transplantation. Am J Transplant. (2006) 6:834–41. doi: 10.1111/j.1600-6143.2006.01266.x
43. Cooney WP, Hentz VR, Breidenbach WC, Jones JW. Successful hand transplantation - one-year follow-up. N Engl J Med. (2001) 343:468–73. doi: 10.1056/NEJM200101043440116
44. Starzl R, Brandacher G, Lee WPA, Carbonell J, Zhang W, Schnider J, et al. Review of the early diagnoses and assessment of rejection in vascularized composite allotransplantation. Clin Dev Immunol. (2013) 2013:1–9. doi: 10.1155/2013/402980
45. Unadkat JV, Schneeberger S, Horibe EH, Goldbach C, Solari MG, Washington KM, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am J Transplant. (2010) 10:251–61. doi: 10.1111/j.1600-6143.2009.02941.x
46. Nadeau KC, Azuma H, Tilney NL. Sequential cytokine dynamics in chronic rejection of rat renal allografts: roles for cytokines RANTES and MCP-1. Proc Natl Acad Sci USA. (1995) 92:8729–33. doi: 10.1073/pnas.92.19.8729
47. Kouwenhoven EA, IJzermans JN, de Bruin RW. Etiology and pathophysiology of chronic transplant dysfunction. Transpl Int. (2000) 13:385–401. doi: 10.1111/j.1432-2277.2000.tb01017.x
48. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. (2014) 14:272–83. doi: 10.1111/ajt.12590
49. Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 meeting report: new concepts in antibody-mediated rejection. Am J Transplant. (2012) 12:563–70. doi: 10.1111/j.1600-6143.2011.03926.x
50. Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. (2013) 257:345–51. doi: 10.1097/SLA.0b013e31826d90bb
51. Morelon E, Petruzzo P, Kanitakis J, Dakpé S, Thaunat O, Dubois V, et al. Face transplantation: partial graft loss of the first case 10 years later. Am J Transplant. (2017) 17:1935–40. doi: 10.1111/ajt.14218
52. Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. (2012) 380:1829–39. doi: 10.1016/S0140-6736(12)61768-1
53. Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. (2012) 380:1819–28. doi: 10.1016/S0140-6736(12)61769-3
54. Guarnera C, Bramanti P, Mazzon E. Alemtuzumab: a review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther Clin Risk Manag. (2017) 13:871–9. doi: 10.2147/TCRM.S134398
55. Lundquist AL, Chari RS, Wood JH, Miller GG, Schaefer HM, Raiford DS, et al. Serum sickness following rabbit antithymocyte-globulin induction in a liver transplant recipient: case report and literature review. Liver Transpl. (2007) 13:647–50. doi: 10.1002/lt.21098
56. Brennan DC, Cibrik D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. (2006) 355:1967–77. doi: 10.1056/NEJMoa060068
57. Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. (2003) 63:2803–35. doi: 10.2165/00003495-200363240-00009
58. Barros VR, Rocha V, Garcia VD, Garcia CD. Anaphylactic shock after retreatment with basiliximab. Transplant Proc. (2003) 35:579. doi: 10.1016/S0041-1345(02)03406-1
59. Sasaki H, Chikaraishi T, Furuhata S, Tsutsumi H, Miyano S, Nakano T, et al. Anaphylactic reaction after initial exposure of basiliximab: case reports. Transplant Proc. (2007) 39:3457–9. doi: 10.1016/j.transproceed.2007.08.104
60. Grinyó J, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti F, Reyes-Acevedo R, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation. (2010) 90:1521–7. doi: 10.1097/TP.0b013e3182007b95
61. Ponticelli C, Glassock RJ. Prevention of complications from use of conventional immunosuppressants: a critical review. J Nephrol. (2019) 32:851–70. doi: 10.1007/s40620-019-00602-5
62. Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. (2000) 16:505–11.
63. Cendales L, Hardy MA. Immunologic considerations in composite tissue transplantation: overview. Microsurgery. (2000) 20:412–9. doi: 10.1002/1098-2752(2000)20:8<412::aid-micr12>3.0.co;2-m
64. Vicari-Christensen M, Repper S, Basile S, Young D. Tacrolimus: review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners' understanding and offer strategies for educating patients and promoting adherence. Prog Transplant. (2009) 19:277–84. doi: 10.1177/152692480901900315
65. Ling Q, Huang H, Han Y, Zhang C, Zhang X, Chen K, et al. The tacrolimus-induced glucose homeostasis imbalance in terms of the liver: from bench to bedside. Am J Transplant. (2019) 20:701–13. doi: 10.1111/ajt.15665
66. Wilusz M, Cieniawski D, Bugajska J, Berska J, Ignacak E, Betkowska-Prokop A, et al. Effects of immunosuppressive drugs on serum fatty acids of phospholipids fraction in renal transplant recipients. Transplant Proc. (2016) 48:1616–22. doi: 10.1016/j.transproceed.2016.03.026
67. Nguyen LS, Vautier M, Allenbach Y, Zahr N, Benveniste O, Funck-Brentano C, et al. Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. (2019) 42:813–25. doi: 10.1007/s40264-019-00810-9
68. Schäffer M, Schier R, Napirei M, Michalski S, Traska T, Viebahn R. Sirolimus impairs wound healing. Langenbecks Arch Surg. (2007) 392:297–303. doi: 10.1007/s00423-007-0174-5
69. Almeida F, Amorim S, Sarmento A, Santos L. Life-threatening everolimus-associated pneumonitis: a case report and a review of the literature. Transplant Proc. (2018) 50:933–8. doi: 10.1016/j.transproceed.2017.12.003
70. Varghese J, Subramanian S, Reddy MS, Shanmugam N, Balajee G, Srinivasan V, et al. Seroprevalence of cytomegalovirus in donors & opportunistic viral infections in liver transplant recipients. Indian J Med Res. (2017) 145:558–62. doi: 10.4103/ijmr.IJMR_1024_14
71. O'Neill HJ, Shirodaria PV. Virus-specific antibodies to Epstein-Barr virus, varicella-zoster virus and rubella virus in renal transplant patients with cytomegalovirus infections. J Infect. (1992) 24:301–9. doi: 10.1016/S0163-4453(05)80035-0
72. Dubernard J-M, Lengelé B, Morelon E, Testelin S, Badet L, Moure C, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. (2007) 357:2451–60. doi: 10.1056/NEJMoa072828
73. Khalifian S, Brazio PS, Mohan R, Shaffer C, Brandacher G, Barth RN, et al. Facial transplantation: the first 9 years. Lancet. (2014) 384:2153–63. doi: 10.1016/S0140-6736(13)62632-X
74. Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation - state of the art. Clin Transplant. (2013) 27:330–7. doi: 10.1111/ctr.12117
75. Gelb BE, Diaz-Siso JR, Plana NM, Jacoby A, Rifkin WJ, Khouri KS, et al. Absence of rejection in a facial allograft recipient with a positive flow crossmatch 24 months after induction with rabbit anti-thymocyte globulin and anti-cd20 monoclonal antibody. Case Rep Transplant. (2018) 2018:7691072. doi: 10.1155/2018/7691072
76. Kueckelhaus M, Fischer S, Seyda M, Bueno EM, Aycart MA, Alhefzi M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl Int. (2016) 29:655–62. doi: 10.1111/tri.12652
77. Landin L, Cavadas PC, Rodriguez-Perez JC, Garcia-Bello MA, Garcia-Cosmes P, Thione A, et al. Improvement in renal function after late conversion to sirolimus-based immunosuppression in composite tissue allotransplantation. Transplantation. (2010) 90:691–2. doi: 10.1097/TP.0b013e3181ebf7ae
78. Goldsmith D, Carrey EA, Edbury S, Smolenski RT, Jagodzinski P, Simmonds HA. Mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase, causes a paradoxical elevation of GTP in erythrocytes of renal transplant patients. Clin Sci. (2004) 107:63–8. doi: 10.1042/CS20030331
79. van der Zwan M, Baan CC, van Gelder T, Hesselink DA. Review of the clinical pharmacokinetics and pharmacodynamics of alemtuzumab and its use in kidney transplantation. Clin Pharmacokinet. (2018) 57:191–207. doi: 10.1007/s40262-017-0573-x
80. Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. (2009) 128:260–70. doi: 10.1111/j.1365-2567.2009.03115.x
81. Kamińska D, Kościelska-Kasprzak K, Myszka M, Banasik M, Chełmoński A, Boratyńska M, et al. Significant infections after hand transplantation in a Polish population. Transplant Proc. (2014) 46:2887–9. doi: 10.1016/j.transproceed.2014.08.028
82. Neuhaus P, Clavien P-A, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double-blind randomized placebo-controlled trial. Liver Transplant. (2002) 8:132–42. doi: 10.1053/jlts.2002.30302
83. Kapic E, Becic F, Kusturica J. Basiliximab, mechanism of action and pharmacological properties. Med Arh. (2004) 58:373–6.
84. Samy KP, Butler JR, Li P, Cooper DKC, Ekser B. The role of costimulation blockade in solid organ and islet xenotransplantation. J Immunol Res. (2017) 2017:8415205. doi: 10.1155/2017/8415205
85. Kinnear G, Jones ND, Wood KJ. Costimulation blockade: current perspectives and implications for therapy. Transplantation. (2013) 95:527–35. doi: 10.1097/TP.0b013e31826d4672
86. Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. (2012) 93:1–10. doi: 10.1097/TP.0b013e31823cab44
87. Huber M, Kemmner S, Renders L, Heemann U. Should belatacept be the centrepiece of renal transplantation? Nephrol Dial Transplant. (2016) 31:1995–2002. doi: 10.1093/ndt/gfw226
88. Muduma G, Hart WM, Patel S, Odeyemi AO. Indirect treatment comparison of belatacept versus tacrolimus from a systematic review of immunosuppressive therapies for kidney transplant patients. Curr Med Res Opin. (2016) 32:1065–72. doi: 10.1185/03007995.2016.1157463
89. Bamgbola O. Metabolic consequences of modern immunosuppressive agents in solid organ transplantation. Ther Adv Endocrinol Metab. (2016) 7:110–27. doi: 10.1177/2042018816641580
90. Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. (2014) 2014:CD010699. doi: 10.1002/14651858.CD010699.pub2
91. Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. (2014) 14:1817–27. doi: 10.1111/ajt.12810
92. Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. (1993) 178:1801–6. doi: 10.1084/jem.178.5.1801
93. Atia A, Moris D, McRae M, Song M, Stempora L, Leopardi F, et al. Th17 cell inhibition in a costimulation blockade based regimen for vascularized composite allotransplantation using a non-human primate model. Transpl Int. (2020) doi: 10.1111/tri.13612. [Epub ahead of print].
94. Foster RD, Pham S, Li S, Aitouche A. Long-term acceptance of composite tissue allografts through mixed chimerism and CD28 blockade. Transplantation. (2003) 76:988–94. doi: 10.1097/01.TP.0000079827.91675.A3
95. Iwasaki N, Gohda T, Yoshioka C, Murakami M, Inobe M, Minami A, et al. Feasibility of immunosuppression in composite tissue allografts by systemic administration of CTLA4Ig. Transplantation. (2002) 73:334–40. doi: 10.1097/00007890-200202150-00004
96. Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. (1996) 381:434–8. doi: 10.1038/381434a0
97. Lin CH, Wang YL, Anggelia MR, Chuang WY, Cheng HY, Mao Q, et al. Combined Anti-CD154/CTLA4Ig costimulation blockade-based therapy induces donor-specific tolerance to vascularized osteomyocutaneous allografts. Am J Transplant. (2016) 16:2030–41. doi: 10.1111/ajt.13694
98. Oh BC, Furtmüller GJ, Fryer ML, Guo Y, Messner F, Krapf J, et al. Vascularized composite allotransplantation combined with costimulation blockade induces mixed chimerism and reveals intrinsic tolerogenic potential. JCI Insight. (2020) 5:e128560. doi: 10.1172/jci.insight.128560
99. Schweizer R, Taddeo A, Waldner M, Klein HJ, Fuchs N, Kamat P, et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am J Transplant. (2020) 20:1272–84. doi: 10.1111/ajt.15726
100. Wachtman GS, Wimmers EG, Gorantla VS, Lin C-H, Schneeberger S, Unadkat JV, et al. Biologics and donor bone marrow cells for targeted immunomodulation in vascularized composite allotransplantation: a translational trial in swine. Transplant Proc. (2011) 43:3541–4. doi: 10.1016/j.transproceed.2011.10.010
101. Krezdorn N, Murakami N, Pomahac B, Riella LV. Immunological characteristics of a patient with belatacept-resistant acute rejection after face transplantation. Am J Transplant. (2016) 16:3305–7. doi: 10.1111/ajt.13977
102. Rostaing L, Vincenti F, Grinyó J, Rice KM, Bresnahan B, Steinberg S, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. (2013) 13:2875–83. doi: 10.1111/ajt.12460
103. Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB, et al. CD57(+) CD4 T cells underlie belatacept-resistant allograft rejection. Am J Transplant. (2016) 16:1102–12. doi: 10.1111/ajt.13613
104. Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB, Iwakoshi NN, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. (2014) 14:59–69. doi: 10.1111/ajt.12526
105. Cendales LC, Xu H, Bacher J, Eckhaus MA, Kleiner DE, Kirk AD. Composite tissue allotransplantation: development of a preclinical model in nonhuman primates. Transplantation. (2005) 80:1447–54. doi: 10.1097/01.tp.0000183292.57349.27
106. Ng ZY, Lellouch AG, Rosales IA, Geoghegan L, Gama A-R, Colvin RB, et al. Graft vasculopathy of vascularized composite allografts in humans: a literature review and retrospective study. Transpl Int. (2019) 32:831–8. doi: 10.1111/tri.13421
107. Ezekian B, Schroder PM, Mulvihill MS, Barbas A, Collins B, Freischlag K, et al. Pretransplant desensitization with costimulation blockade and proteasome inhibitor reduces DSA and delays antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients. J Am Soc Nephrol. (2019) 30:2399–411. doi: 10.1681/ASN.2019030304
108. Everly MJ, Roberts M, Townsend R, Bray RA, Gebel HM. Comparison of de novo IgM and IgG anti-HLA DSAs between belatacept- and calcineurin-treated patients: an analysis of the BENEFIT and BENEFIT-EXT trial cohorts. Am J Transplant. (2018) 18:2305–13. doi: 10.1111/ajt.14939
109. Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. (2018) 18:1783–9. doi: 10.1111/ajt.14721
110. Weissenbacher A, Loupy A, Chandraker A, Schneeberger S. Donor-specific antibodies and antibody-mediated rejection in vascularized composite allotransplantation. Curr Opin Organ Transplant. (2016) 21:510–5. doi: 10.1097/MOT.0000000000000349
111. Karpe KM, Talaulikar GS, Walters GD. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev. (2017) 7:CD006750. doi: 10.1002/14651858.CD006750.pub2
112. Charlton M, Levitsky J, Aqel B, O'Grady J, Hemibach J, Rinella M, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. (2018) 102:727–43. doi: 10.1097/TP.0000000000002147
113. Penninga L, Møller CH, Gustafsson F, Gluud C, Steinbrüchel DA. Immunosuppressive T-cell antibody induction for heart transplant recipients. Cochrane Database Syst Rev. (2013) 12:CD008842. doi: 10.1002/14651858.CD008842.pub2
114. Cendales L, Levine M, Bartlett S, Cheeseman J, Drachenberg C, Hancock W, et al. Skin as a harbinger of rejection of underlying structures in vascularized composite allografts: concordance or discordance? Am J Transplant. (2016) 16(Suppl. 3):433. Available online at: https://scholars.duke.edu/display/pub1167999
115. Kanitakis J. The challenge of dermatopathological diagnosis of composite tissue allograft rejection: a review. J Cutan Pathol. (2008) 35:738–44. doi: 10.1111/j.1600-0560.2007.00889.x
116. Noble J, Jouve T, Janbon B, Rostaing L, Malvezzi P. Belatacept in kidney transplantation and its limitations. Expert Rev Clin Immunol. (2019) 15:359–67. doi: 10.1080/1744666X.2019.1574570
117. Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. (1999) 104:1715–22. doi: 10.1172/JCI8082
118. Gilson CR, Milas Z, Gangappa S, Hollenbaugh D, Pearson TC, Ford ML, et al. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J Immunol. (2009). 183:1625–35. doi: 10.4049/jimmunol.0900339
119. Riella LV, Sayegh MH. T-cell co-stimulatory blockade in transplantation: two steps forward one step back! Expert Opin Biol Ther. (2013) 13:1557–68. doi: 10.1517/14712598.2013.845661
120. Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu X-Z. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. (2011) 117:3096–103. doi: 10.1182/blood-2010-08-301275
121. Mathews DV, Wakwe WC, Kim SC, Lowe MC, Breeden C, Roberts ME, et al. Belatacept-resistant rejection is associated with CD28+ memory CD8 T cells. Am J Transplant. (2017) 17:2285–99. doi: 10.1111/ajt.14349
122. Mathews DV, Dong Y, Higginbotham LB, Kim SC, Breeden CP, Stobert EA, et al. CD122 signaling in CD8+ memory T cells drives costimulation-independent rejection. J Clin Invest. (2018) 128:4557–72. doi: 10.1172/JCI95914
123. Xu H, Mehta AK, Gao Q, Lee H-J, Ghali A, Guasch A, et al. B cell reconstitution following alemtuzumab induction under a belatacept-based maintenance regimen. Am J Transplant. (2020) 20:653–62. doi: 10.1111/ajt.15639
124. Castro-Rojas CM, Godarova A, Shi T, Hummel SA, Shields A, Tremblay S, et al. mTOR inhibitor therapy diminishes circulating CD8+ CD28- effector memory T cells and improves allograft inflammation in belatacept-refractory renal allograft rejection. Transplantation. (2020) 104:1058–69. doi: 10.1097/TP.0000000000002917
125. Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. (2012) 12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x
126. Vanhove B, Poirier N, Soulillou J-P, Blancho G. Selective costimulation blockade with antagonist anti-CD28 therapeutics in transplantation. Transplantation. (2019) 103:1783–9. doi: 10.1097/TP.0000000000002740
Keywords: vascularized composite allotransplantation, belatacept, costimulation blockade, VCA, hand transplantation
Citation: Giannis D, Moris D and Cendales LC (2020) Costimulation Blockade in Vascularized Composite Allotransplantation. Front. Immunol. 11:544186. doi: 10.3389/fimmu.2020.544186
Received: 19 March 2020; Accepted: 19 August 2020;
Published: 17 September 2020.
Edited by:
Gilles Blancho, Université de Nantes, FranceReviewed by:
Emmanuel Morelon, Hospices Civils de Lyon, FranceCarl Atkinson, Medical University of South Carolina, United States
Copyright © 2020 Giannis, Moris and Cendales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda C. Cendales, linda.cendales@duke.edu
†These authors have contributed equally to this work
 Dimitrios Giannis
Dimitrios Giannis Dimitrios Moris
Dimitrios Moris Linda C. Cendales
Linda C. Cendales