Abstract
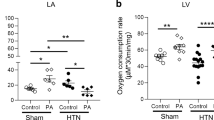
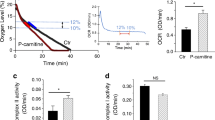
Nitric oxide (NO) affects mitochondrial activity through its interactions with complexes. Here, we investigated regulations of complex I (C-I) and complex II (C-II) by neuronal NO synthase (nNOS) in the presence of fatty acid supplementation and the impact on left ventricular (LV) mitochondrial activity from sham and angiotensin II (Ang-II)–induced hypertensive (HTN) rats. Our results showed that nNOS protein was expressed in sham and HTN LV mitochondrial enriched fraction. In sham, oxygen consumption rate (OCR) and intracellular ATP were increased by palmitic acid (PA) or palmitoyl-carnitine (PC). nNOS inhibitor, S-methyl-l-thiocitrulline (SMTC), did not affect OCR or cellular ATP increment by PA or PC. However, SMTC increased OCR with PA + malonate (a C-II inhibitor), but not with PA + rotenone (a C-I inhibitor), indicating that nNOS attenuates C-I with fatty acid supplementation. Indeed, SMTC increased C-I activity but not that of C-II. Conversely, nNOS-derived NO was increased by rotenone + PA in LV myocytes. In HTN, PC increased the activity of C-I but reduced that of C-II, consequently OCR was reduced. SMTC increased both C-I and C-II activities with PC, resulted in OCR enhancement in the mitochondria. Notably, SMTC increased OCR only with rotenone, suggesting that nNOS modulates C-II-mediated OCR in HTN. nNOS-derived NO was partially reduced by malonate + PA. Taken together, nNOS attenuates C-I-mediated mitochondrial OCR in the presence of fatty acid in sham and C-I modulates nNOS activity. In HTN, nNOS attenuates C-I and C-II activities whereas interactions between nNOS and C-II maintain mitochondrial activity.







Similar content being viewed by others
References
Arrell DK, Elliott ST, Kane LA, Guo YR, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE (2006) Proteomic analysis of pharmacological preconditioning - novel protein targets converge to mitochondrial metabolism pathways. Circ Res 99:706–714. https://doi.org/10.1161/01.RES.0000243995.74395.f8
Beltran B, Quintero M, Garcia-Zaragoza E, O’Connor E, Esplugues JV, Moncada S (2002) Inhibition of mitochondrial respiration by endogenous nitric oxide: a critical step in Fas signaling. Proc Natl Acad Sci U S A 99:8892–8897. https://doi.org/10.1073/pnas.092259799
Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL, Heymes C (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110:2368–2375. https://doi.org/10.1161/01.Cir.0000145160.04084.Ac
Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF (2005) Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435:502–506. https://doi.org/10.1038/nature03527
Bombicino SS, Iglesias DE, Zaobornyj T, Boveris A, Valdez LB (2016) Mitochondrial nitric oxide production supported by reverse electron transfer. Arch Biochem Biophys 607:8–19. https://doi.org/10.1016/j.abb.2016.08.010
Boveris A, Arnaiz SL, Bustamante J, Alvarez S, Valdez L, Boveris AD, Navarro A (2002) Pharmacological regulation of mitochondrial nitric oxide synthase. Methods Enzymol 359:328–339. https://doi.org/10.1016/s0076-6879(02)59196-5
Burkard N, Czolbe M, Williams T, Frantz S, Hofmann U, Ritter O (2010) Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia-reperfusion. Circulation 122:1588–1603
Davidson SM, Duchen MR (2006) Effects of NO on mitochondrial function in cardiomyocytes: pathophysiological relevance. Cardiovasc Res 71:10–21. https://doi.org/10.1016/j.cardiores.2006.01.019
Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B (2005) nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation 112:3729–3737. https://doi.org/10.1161/Circulationaha.105.539437
Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE (2014) Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20:281–294. https://doi.org/10.1089/ars.2012.4918
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Douette P, Sluse FE (2006) Mitochondrial uncoupling proteins: new insights from functional and proteomic studies. Free Radical Bio Med 40:1097–1107. https://doi.org/10.1016/j.freeradbiomed.2005.12.010
Genova ML, Lenaz G (2014) Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 1837:427–443. https://doi.org/10.1016/j.bbabio.2013.11.002
Jang JH, Chun JN, Godo S, Wu G, Shimokawa H, Jin CZ, Jeon JH, Kim SJ, Jin ZH, Zhang YH (2015) ROS and endothelial nitric oxide synthase (eNOS)-dependent trafficking of angiotensin II type 2 receptor begets neuronal NOS in cardiac myocytes. Basic Res Cardiol 110:21. https://doi.org/10.1007/s00395-015-0477-6
Jin CZ, Jang JH, Wang Y, Kim JG, Bae YM, Shi J, Che CR, Kim SJ, Zhang YH (2012) Neuronal nitric oxide synthase is up-regulated by angiotensin II and attenuates NADPH oxidase activity and facilitates relaxation in murine left ventricular myocytes. J Mol Cell Cardiol 52:1274–1281. https://doi.org/10.1016/j.yjmcc.2012.03.013
Jin CZ, Jang JH, Kim HJ, Wang Y, Hwang IC, Sadayappan S, Park BM, Kim SH, Jin ZH, Seo EY, Kim KH, Kim YJ, Kim SJ, Zhang YH (2013) Myofilament Ca2+ desensitization mediates positive lusitropic effect of neuronal nitric oxide synthase in left ventricular myocytes from murine hypertensive heart. J Mol Cell Cardiol 60:107–115. https://doi.org/10.1016/j.yjmcc.2013.04.017
Jin CL, Yin MZ, Paeng JC, Ha S, Lee JH, Jin P, Jin CZ, Zhao ZH, Wang Y, Kang KW, Leem CH, Park JW, Kim SJ, Zhang YH (2017) Neuronal nitric oxide synthase modulation of intracellular Ca(2+) handling overrides fatty acid potentiation of cardiac inotropy in hypertensive rats. Pflugers Arch 469:1359–1371. https://doi.org/10.1007/s00424-017-1991-1
Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Peterson J (2001) Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A 98:14126–14131. https://doi.org/10.1073/pnas.241380298
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258. https://doi.org/10.1152/physrev.00015.2009
Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T (2000) Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol 20:7311–7318. https://doi.org/10.1128/Mcb.20.19.7311-7318.2000
Parihar MS, Nazarewicz RR, Kincaid E, Bringold U, Ghafourifar P (2008) Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochem Biophys Res Commun 366:23–28. https://doi.org/10.1016/j.bbrc.2007.11.056
Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Archives of Biochemistry and Biophysics 328:85–92. https://doi.org/10.1006/abbi.1996.0146
Schafer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, Vonck J (2006) Architecture of active mammalian respiratory chain supercomplexes. J Biol Chem 281:15370–15375. https://doi.org/10.1074/jbc.M513525200
Scialo F, Fernandez-Ayala DJ, Sanz A (2017) Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol 8:428. https://doi.org/10.3389/fphys.2017.00428
Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res 92:E52–E59. https://doi.org/10.1161/01.Res.0000064585.95749.6d
Walker JE (1992) The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys 25:253–324. https://doi.org/10.1017/s003358350000425x
Walker JE, Arizmendi JM, Dupuis A, Fearnley IM, Finel M, Medd SM, Pilkington SJ, Runswick MJ, Skehel JM (1992) Sequences of 20 subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Application of a novel strategy for sequencing proteins using the polymerase chain reaction. J Mol Biol 226:1051–1072
Weiss JN, Korge P, Honda HM, Ping PP (2003) Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93:292–301. https://doi.org/10.1161/01.Res.0000087542.26971.D4
Wilson FH, Hariri A, Farhi A, Zhao HY, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian CM, Shulman GI, Scheinman SJ, Lifton RP (2004) A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 306:1190–1194. https://doi.org/10.1126/science.1102521
Zaobornyj T, Valdez LB, Iglesias DE, Gasco M, Gonzales GF, Boveris A (2009) Mitochondrial nitric oxide metabolism during rat heart adaptation to high altitude: effect of sildenafil, L-NAME, and L-arginine treatments. Am J Physiol Heart Circ Physiol 296:H1741–H1747. https://doi.org/10.1152/ajpheart.00422.2008
Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B (2008) Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 102:242–249. https://doi.org/10.1161/Circresaha.107.164798
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. https://doi.org/10.1152/physrev.00026.2013
Funding
This work was financially supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2C1005720), National Natural Science Foundation of China (NSFC 31660284, NSFC31860288) and Seoul National University Research Grant 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y.N., Sudarshan, V.K., Zhu, S.C. et al. Functional interactions between complex I and complex II with nNOS in regulating cardiac mitochondrial activity in sham and hypertensive rat hearts. Pflugers Arch - Eur J Physiol 472, 1743–1755 (2020). https://doi.org/10.1007/s00424-020-02458-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02458-2