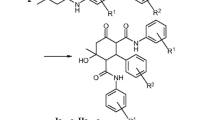
Abstract
Cascade reaction of 2 equiv. of furfural (or equimolar amounts of furfural and aromatic aldehyde) with secondary amines and ethyl cyanoacetate afforded diethyl esters of 8-(dialkylamino)-3-aryl-6-oxo-2,4-dicyanobicyclo[3.2.1]octane-2,4-dicarboxylic acids with yields of 37–54%. Antimicrobial activity of a number of obtained compounds in vitro was studied, and biological activity in silico was analyzed. The obtained bicyclo[3.2.1]octanes are inactive or exhibit weak fungicidal activity, but exhibit moderate bactericidal effect.



Similar content being viewed by others
REFERENCES
Filippini, M.H. and Rodriguez, J., Chem. Rev., 1999, vol. 99, p. 27. https://doi.org/10.1021/cr970029u
Presset, M., Coquerel, Y., and Rodriguez, J., Chem. Rev., 2013, vol. 113, p. 525. https://doi.org/10.1021/cr200364p
Lefranc, A., Gremaud, L., and Alexakis, A., Org. Lett., 2014, vol. 16. N 20, p. 5242. https://doi.org/10.1021/ol502171h
He, C., Bai, Z., Hu, J., Wang, B., Xie, H., Yu, L., and Ding, H., Chem. Commun., 2017, vol. 53, p. 8435. https://doi.org/10.1039/c7cc04292b
Takatori, K., Ota, S., Tendo, K., Matsunaga, K., Nagasawa, K., Watanabe, S., Kishida, A., Kogen, H., and Nagaoka, H., Org. Lett., 2017, vol. 19, p. 3763. https://doi.org/10.1021/acs.orglett.7b01604
Zhao, J., Yang, S., Xie, X., Li, X., and Liu, Y., J. Org. Chem., 2018, vol. 83, no. 3, p. 1287. https://doi.org/10.1021/acs.joc.7b02816
Yuan, Z., Feng, Z., Zeng, Y., Zhao, X., Lin, A., and Yao, H., Angew. Chem., 2019, vol. 131, no. 9, p. 2910. https://doi.org/10.1002/ange.201900059
Meltzer, P.C., Blundell, P., Yong, Y.F., Chen, Z., George, C., Gonzalez, M.D., and Madras, B.K., J. Med. Chem., 2000, vol. 43, no. 16, p. 2982. https://doi.org/10.1021/jm000191g
Meltzer, P.C., Blundell, P., Chen, Z., Yong, Y.F., and Madras, B.K., Bioorg. Med. Chem. Lett., 1999, vol. 9, p. 857. https://doi.org/10.1016/S0960-894X(99)00098-0
Zefirov, N.A., Lavrushkina, E.A., Kuznetsov, S.A., and Zefirova, O.N., Biomed. Khim., 2019, vol. 65, no. 2, p. 86. https://doi.org/10.18097/PBMC20196502086
Santos, M.F., Alcântara, B.G., Feliciano, C.D.R., Silva, A.F., Maiolini, T.C., Neto, A.K., Murgu, M., Chagas de Paula, D.A., and Soares, M.G., Phytochem. Lett., 2019, vol. 30, p. 31. https://doi.org/10.1016/j.phytol.2019.01.014
Liu, Y., Liu, F., Qiao, M.M., Guo, L., Chen, M.H., Peng, C., and Xiong, L., Org. Lett., 2019, vol. 21, no. 4, p. 1197. https://doi.org/10.1021/acs.orglett.9b00149
Peter, C., Geoffroy, P., and Miesch, M., Org. Biomol. Chem., 2018, vol. 16, p. 1381. https://doi.org/10.1039/c7ob03124f
Dotsenko, V.V., Ismiev, A.I., Khrustaleva, A.N., Frolov, K.A., Krivokolysko, S.G., Chigorina, E.A., Snizhko, A.P., Gromenko, V.M., Bushmarinov, I.S., Askerov, R.K., Pekhtereva, T.M., Suykov, S.Yu., Papayanina, E.S., Mazepa, A.V., and Magerramov, A.M., Chem. Heterocycl. Compd., 2016, vol. 52, no. 7, p. 473. https://doi.org/10.1007/s10593-016-1918-3
Hajiyeva, K., Ismiev, A., Franz, M., Schmidtmann, M., Martens, J., and Maharramov, A., Synth. Commun., 2017, vol. 47, no. 22, p. 2031. https://doi.org/10.1080/00397911.2017.1359845
Dotsenko, V.V., Frolov, K.A., Pekhtereva, T.M., Papaianina, O.S., Suykov, S.Yu., and Krivokolysko, S.G., ACS Comb. Sci., 2014, vol. 16, no. 10, p. 543. https://doi.org/10.1021/co5000807
Ismiev, A.I., Dotsenko, V.V., Aksenov, N.A., Mamedova, G.Z., and Magerramov, A.M., Russ. J. Gen. Chem., 2018, vol. 88, no. 7, p. 1533. https://doi.org/10.1134/S1070363218070289
Ismiyev, A.I., Dotsenko, V.V., Bespalov, A.V., Netreba, E.E., and Maharramov, A.M., Russ. J. Gen. Chem., 2020, vol. 90, no. 2, p. 187. https://doi.org/10.1134/S1070363220020048
Piutti, C. and Quartieri, F., Molecules, 2013, vol. 18, no. 10, p. 12290. https://doi.org/10.3390/molecules181012290
Verrier, C., Moebs-Sanchez, S., Queneau, Y., and Popowycz, F., Org. Biomol. Chem., 2018, vol. 16, no. 5, p. 676. https://doi.org/10.1039/C7OB02962D
Piancatelli, G., D’Auria, M., and D’Onofrio, F., Synthesis, 1994, no. 9, p. 867. https://doi.org/10.1055/s-1994-25591
Tius, M.A., Eur. J. Org. Chem., 2005, p. 2193. https://doi.org/10.1002/ejoc.200500005
Gomes, R.F., Coelho, J.A., and Afonso, C.A., Chem. Eur. J., 2018, vol. 24, no. 37, p. 9170. https://doi.org/10.1002/chem.201705851
Li, H., Tong, R., and Sun, J., Angew. Chem. Int. Ed., 2016, vol. 55, no. 48, p. 15125. https://doi.org/10.1002/anie.201607714
Tang, W.B., Cao, K.S., Meng, S.S., and Zheng, W.H., Synthesis, 2017, vol. 49, no. 16, p. 3670. https://doi.org/10.1055/s-0036-1589040
Palmer, L.I. and de Alaniz, J.R., Synlett., 2014, vol. 25, no. 1, p. 8. https://doi.org/10.1055/s-0033-1340157
Nardi, M., Costanzo, P., De Nino, A., Di Gioia, M.L., Olivito, F., Sindona, G., and Procopio, A., Green Chem., 2017, vol. 19, no. 22, p. 5403. https://doi.org/10.1039/C7GC02303K
Way2Drug. antiBac-Pred, Laboratory for StructureFunction Based Drug Design, Institute of Biomedical Chemistry (IBMC), Moscow, Russia. http://way2drug.com/antibac/.
Filimonov, D.A., Lagunin, A.A., Gloriozova, T.A., Rudik, A.V., Druzhilovskii, D.S., Pogodin, P.V., and Poroikov, V.V., Chem. Heterocycl. Compd., 2014, vol. 50, no. 3, p. 444. https://doi.org/10.1007/s10593-014-1496-1
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug. Deliv. Rev., 1997, vol. 23, nos. 1–3, p. 4. https://doi.org/10.1016/S0169-409X(96)00423-1
Lipinski, C.A., Drug Discov. Today: Technologies, 2004, vol. 1, no. 4, p. 337. https://doi.org/10.1016/j.ddtec.2004.11.007
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug. Deliv. Rev., 2012, vol. 64, p. 4. https://doi.org/10.1016/j.addr.2012.09.019
Sander, T., OSIRIS Property Explorer, Idorsia Pharmaceuticals Ltd., Switzerland. http://www.organic-chemistry.org/prog/peo/.
PASS Online, Laboratory for Structure-Function Based Drug Design, Institute of Biomedical Chemistry (IBMC), Moscow, Russia. http://www.pharmaexpert.ru/passonline/predict.php.
Molinspiration Property Calculation Service, Molinspiration Cheminformatics, Slovak Republic, 2002. https://www.molinspiration.com/.
Daina, A., Michielin, O., and Zoete, V., Sci. Rep., 2017, vol. 7, article no. 42717. https://doi.org/10.1038/srep42717
Gfeller, D., Grosdidier, A., Wirth, M., Daina, A., Michielin, O., and Zoete, V., Nucl. Acids Res., 2014, vol. 42, no. W1, p. W32. https://doi.org/10.1093/nar/gku293
Cheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., Lee, P.W., and Tang, Y., J. Chem. Inf. Model., 2012, vol. 52, no. 11, p. 3099. https://doi.org/10.1021/ci300367a
Balouiri, M., Sadiki, M., and Ibnsouda, S.K., J. Pharm. Analysis, 2016, vol. 6, no. 2, p. 71. https://doi.org/10.1016/j.jpha.2015.11.005
Funding
This work was financially supported by the Ministry of Education and Science of the Russian Federation (project no. 0795-2020-0031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Ismiyev, A.I., Shoaib, M., Dotsenko, V.V. et al. Synthesis and Biological Activity of 8-(Dialkylamino)-3-aryl-6-oxo-2,4-dicyanobicyclo[3.2.1]octane-2,4-dicarboxylic Acids Diethyl Esters. Russ J Gen Chem 90, 1418–1425 (2020). https://doi.org/10.1134/S1070363220080071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220080071