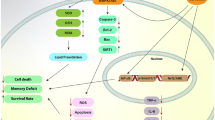
Abstract
Neurodegenerative disorders are characterized by mitochondrial dysfunction and subsequently oxidative stress, inflammation, and apoptosis that contribute to neuronal cytotoxicity and degeneration. Recent studies reported that crocin, a carotenoid chemical compound common in crocus and gardenia flowers, has protective effects in neurodegenerative disorders due to its anti-oxidative, anti-inflammatory, and anti-apoptotic properties in the nervous system. This article reviews the new experimental, clinical, and pharmacological studies on the neuroprotective properties of crocin and its potential mechanisms to modulate metabolic oxidative stress and inflammation in neurodegenerative disorders.






Similar content being viewed by others
References
Najas-García A, Rufián S, Rojo E (2014) Neurodevelopment or neurodegeneration: review of theories of schizophrenia. Actas Esp Psiquiatr 42:185–195
Krantic S, Mechawar N, Reix S, Quirion R (2005) Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci 28:670–676
Muchowski PJ (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35:9–12
Campbell A (2004) Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci 1035:117–132
Rodríguez MJ, Pugliese M, Mahy N (2009) Drug abuse, brain calcification and glutamate-induced neurodegeneration. Current Drug Abuse Rev 2:99–112
Farber NB, Olney JW (2003) Drugs of abuse that cause developing neurons to commit suicide. Dev Brain Res 147:37–45
Büttner A (2014) The neuropathology of drug abuse. In: The effects of drug abuse on the human nervous system. Elsevier, Amsterdam, pp 169–202
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585:3715–3723
Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6:11
Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Investig 115:1449–1457
Yates D (2012) Neurodegenerative disease: neurodegenerative networking. Nat Rev Neurosci 13:288
Floyd RA, Hensley K (2002) Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 23:795–807
Amor S, Puentes F, Baker D, Van Der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129:154–169
Griffin WST (2006) Inflammation and neurodegenerative diseases. Am J Clin Nutr 83:470S–474S
Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348:1365–1375
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimer's Dis 42:S125–S152
Ekshyyan O, Aw TY (2004) Apoptosis: a key in neurodegenerative disorders. Curr Neurovasc Res 1:355–371
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787
Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P (2015) Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol 11:11
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA et al (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8:2003
Trushina E, McMurray C (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145:1233–1248
Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK et al (2011) Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol 187:4788–4799
Farkhondeh T, Samarghandian S, Yazdi HS, Samini F (2018) The protective effects of crocin in the management of neurodegenerative diseases: a review. Am J Neurodegen Dis 7:1
Kim H (2005) Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol Res 27:287–301
Kumar GP, Khanum F (2012) Neuroprotective potential of phytochemicals. Pharmacogn Rev 6:81
Hosseinzadeh H, Talebzadeh F (2005) Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 76:722–724
Lee J, Kim C-H, Simon DK, Aminova LR, Andreyev AY, Kushnareva YE et al (2005) Mitochondrial cyclic AMP response element-binding protein (CREB) mediates mitochondrial gene expression and neuronal survival. J Biol Chem 280:40398–40401
Yousefsani BS, Mehri S, Pourahmad J, Hosseinzadeh H (2018) Crocin prevents sub-cellular organelle damage, proteolysis and apoptosis in rat hepatocytes: a justification for its hepatoprotection (Spring 2018). Iran J Pharm Res 17(2):553
Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y et al (2008) Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: a relationship investigation between antioxidant activity and crocin contents. Food Chem 109:484–492
Khalili M, Roghani M, Ekhlasi M (2010) The effect of aqueous Crocus sativus L. extract on intracerebroventricular streptozotocin-induced cognitive deficits in rat: a behavioral analysis. Iran J Pharm Res 8:185–191
Singla RK, Bhat G (2011) Crocin: an overview. Indo Glob J Pharm Sci 1:281–286
Alavizadeh SH, Hosseinzadeh H (2014) Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol 64:65–80
Nam KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U et al (2010) Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 648:110–116
Sarshoori JR, Asadi MH, Mohammadi MT (2014) Neuroprotective effects of crocin on the histopathological alterations following brain ischemia-reperfusion injury in rat. Iran J Basic Med Sci 17:895
Soeda S, Ochiai T, Shimeno H, Saito H, Abe K, Tanaka H et al (2007) Pharmacological activities of crocin in saffron. J Nat Med 61:102–111
Wang K, Zhang L, Rao W, Su N, Hui H, Wang L et al (2015) Neuroprotective effects of crocin against traumatic brain injury in mice: involvement of notch signaling pathway. Neurosci Lett 591:53–58
Qi Y, Chen L, Zhang L, Liu W-B, Chen X-Y, Yang X-G (2013) Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res 107:44–51
Heidari S, Mehri S, Hosseinzadeh H (2017) Memory enhancement and protective effects of crocin against D-galactose aging model in the hippocampus of Wistar rats. Iran J Basic Med Sci 20:1250
Bandegi AR, Rashidy-Pour A, Vafaei AA, Ghadrdoost B (2014) Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull 4:493
Zhang X-y, Zhang X-j, Xv J, Jia W, Pu X-y, Wang H-y et al (2018) Crocin attenuates acute hypobaric hypoxia-induced cognitive deficits of rats. Eur J Pharmacol 818:300–305
Akbari-Fakhrabadi M, Najafi M, Mortazavian S, Rasouli M, Memari AH, Shidfar F (2019) Effect of saffron (Crocus sativus L.) and endurance training on mitochondrial biogenesis, endurance capacity, inflammation, antioxidant, and metabolic biomarkers in Wistar rats. J Food Biochem 43:e12946
Chen L, Qi Y, Yang X (2015) Neuroprotective effects of crocin against oxidative stress induced by ischemia/reperfusion injury in rat retina. Ophthalmic Res 54:157–168
Srivastava R, Ahmed H, Dixit R (2010) Crocus sativus L.: a comprehensive review. Pharmacogn Rev 4:200
Bostan HB, Mehri S, Hosseinzadeh H (2017) Toxicology effects of saffron and its constituents: a review. Iran J Basic Med Sci 20:110
Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10:S18
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267:4904–4911
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322:254–262
Shukla V, Mishra SK, Pant HC (2011) Oxidative stress in neurodegeneration. Adv Pharmacol Sci. https://doi.org/10.1155/2011/572634
Sultana R, Perluigi M, Butterfield DA (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169
Reed TT (2011) Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med 51:1302–1319
Arlt S, Beisiegel U, Kontush A (2002) Lipid peroxidation in neurodegeneration: new insights into Alzheimer's disease. Curr Opin Lipidol 13:289–294
Farooqui AA, Ong W-Y, Horrocks LA (2004) Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A 2. Neurochem Res 29:1961–1977
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discovery 3:205
Johnson WM, Wilson-Delfosse AL, Mieyal J (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4:1399–1440
Sabens Liedhegner EA, Gao X-H, Mieyal JJ (2012) Mechanisms of altered redox regulation in neurodegenerative diseases—focus on S-glutathionylation. Antioxid Redox Signal 16:543–566
Bailey SM, Patel VB, Young TA, Asayama K, Cunningham CC (2001) Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res 25:726–733
Lin S-C, Lin C-H, Lin C-C, Lin Y-H, Chen C-F, Chen I-C et al (2002) Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J Biomed Sci 9:401–409
Yuan L, Kaplowitz N (2009) Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med 30:29–41
Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM et al (2004) Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res 28:173–181
Klein JA, Ackerman SL (2003) Oxidative stress, cell cycle, and neurodegeneration. J Clin Investig 111:785–793
Ruszkiewicz J, Albrecht J (2015) Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem Int 88:66–72
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6:337–350
Cruz R, Almaguer WM, Bergado JR (2003) Glutathione in cognitive function and neurodegeneration. Revista de Neurologia 36:877–886
Cardoso BR, Hare DJ, Bush AI, Roberts BR (2017) Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry 22:328–335
Aoyama K, Nakaki T (2013) Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci 14:21021–21044
Jóhannesson T, Kristinsson J, Snaedal J (2003) Neurodegenerative diseases, antioxidative enzymes and copper. A review of experimental research. Laeknabladid 89:659–671
Sánchez-Valle V, Chavez-Tapia NC, Uribe M, Méndez-Sánchez N (2012) Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem 19:4850–4860
Han D, Hanawa N, Saberi B, Kaplowitz N (2006) Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol-Gastrointest Liver Physiol 291:G1–G7
Zeviani M, Carelli V (2007) Mitochondrial disorders. Curr Opin Neurol 20:564–571
Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L (2006) Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimer's Dis 10:59–73
Lezi E, Swerdlow RH (2012) Mitochondria in neurodegeneration. In: Advances in mitochondrial medicine. Springer, Boston, pp 269–286
Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14:457–487
Orth M, Schapira A (2001) Mitochondria and degenerative disorders. Am J Med Genet 106:27–36
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342:619–630
Itoh K, Nakamura K, Iijima M, Sesaki H (2013) Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23:64–71
Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O et al (2011) Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol 69:481–492
Reeve AK, Krishnan KJ, Turnbull D (2008) Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann N Y Acad Sci 1147:21–29
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9:505
Prieto M, Vázquez JA, Murado M (2015) Crocin bleaching antioxidant assay revisited: application to microplate to analyse antioxidant and pro-oxidant activities. Food Chem 167:299–310
Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F et al (2011) Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol 667:222–229
Motaghinejad M, Safari S, Feizipour S, Sadr S (2019) Crocin may be useful to prevent or treatment of alcohol induced neurodegeneration and neurobehavioral sequels via modulation of CREB/BDNF and Akt/GSK signaling pathway. Med Hypotheses 124:21–25
Thushara R, Hemshekhar M, Santhosh MS, Jnaneshwari S, Nayaka S, Naveen S et al (2013) Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol Cell Biochem 373:73–83
Assimopoulou A, Sinakos Z, Papageorgiou V (2005) Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res 19:997–1000
Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H (2012) Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol 32:227–235
Mehri S, Abnous K, Khooei A, Mousavi SH, Shariaty VM, Hosseinzadeh H (2015) Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci 18:902
Mousavi SH, Tayarani N, Parsaee H (2010) Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol 30:185–191
Hosseinzadeh H, Shamsaie F, Mehri S (2009) Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents, crocin and safranal. Pharmacogn Mag 5:419
Hosseinzadeh H, Modaghegh MH, Saffari Z (2009) Crocus sativus L. (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid-Based Complement Altern Med 6:343–350
Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA (2012) Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res 26:381–386
Ochiai T, Soeda S, Ohno S, Tanaka H, Shoyama Y, Shimeno H (2004) Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem Int 44:321–330
Mohammadzadeh L, Hosseinzadeh H, Abnous K, Razavi BM (2018) Neuroprotective potential of crocin against malathion-induced motor deficit and neurochemical alterations in rats. Environ Sci Pollut Res 25:4904–4914
Tundis R, Loizzo MR, Nabavi SM, Orhan IE, Skalicka-Woźniak K, D’Onofrio G et al (2018) Natural compounds and their derivatives as multifunctional agents for the treatment of Alzheimer disease. In: Discovery and development of neuroprotective agents from natural products. Elsevier, Amsterdam, pp 63–102
Soeda S, Ochiai T, Paopong L, Tanaka H, Shoyama Y, Shimeno H (2001) Crocin suppresses tumor necrosis factor-α-induced cell death of neuronally differentiated PC-12 cells. Life Sci 69:2887–2898
Zheng Y-Q, Liu J-X, Wang J-N, Xu L (2007) Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res 1138:86–94
Soeda S, Aritake K, Urade Y, Sato H, Shoyama Y (2016) Neuroprotective activities of saffron and crocin. In: The benefits of natural products for neurodegenerative diseases. Springer, Boston, pp 275–292
Rajaei Z, Hosseini M, Alaei H (2016) Effects of crocin on brain oxidative damage and aversive memory in a 6-OHDA model of Parkinson’s disease. Arq Neuropsiquiatr 74:723–729
Oruc S, Gönül Y, Tunay K, Oruc OA, Bozkurt MF, Karavelioğlu E et al (2016) The antioxidant and antiapoptotic effects of crocin pretreatment on global cerebral ischemia reperfusion injury induced by four vessels occlusion in rats. Life Sci 154:79–86
Meamarbashi A, Rajabi A (2016) Potential ergogenic effects of saffron. J Diet Suppl 13:522–529
Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H (2004) Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α-tocopherol. Neurosci Lett 362:61–64
Korani S, Korani M, Sathyapalan T, Sahebkar A (2019) Therapeutic effects of crocin in autoimmune diseases: a review. BioFactors 45(6):835–843
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7:161
Hennessy E, Griffin ÉW, Cunningham C (2015) Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1β and TNFα. J Neurosci 35:8411–8422
Murray CL, Skelly DT, Cunningham C (2011) Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6. J Neuroinflamm 8:50
Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2:734
Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat Inflamm. https://doi.org/10.1155/2013/480739
Olson L, Humpel C (2010) Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer’s disease and mild cognitive impairment. Exp Gerontol 45:41–46
Joo J, Lee M, Bae S, An SSA (2013) Blood biomarkers: from nanotoxicity to neurodegeneration. SPIE Newsroom
Kempuraj D, Thangavel R, Natteru P, Selvakumar G, Saeed D, Zahoor H et al (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1:1003
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7:354–365
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35:419–432
Schwartz M, Shechter R (2010) Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol 6:405
Brown GC, Bal-Price A (2003) Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 27:325–355
Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J (2010) Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10:411–418
Di Filippo M, Chiasserini D, Tozzi A, Picconi B, Calabresi P (2010) Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimer's Dis 20:S369–S379
Vodret S, Bortolussi G, Jašprová J, Vitek L, Muro AF (2017) Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1-/-mouse model. J Neuroinflamm 14:64
Escudero-Lourdes C (2016) Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: role of oxidative stress and inflammatory responses. Neurotoxicology 53:223–235
Sun B, Karin M (2008) NF-κB signaling, liver disease and hepatoprotective agents. Oncogene 27:6228
Gh Popescu BF, Nichol H (2011) Mapping brain metals to evaluate therapies for neurodegenerative disease. CNS Neurosci Ther 17:256–268
Zhang L, Previn R, Lu L, Liao R-F, Jin Y, Wang R-K (2018) Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull 142:352–359
Li X, Jiang C, Zhu W (2017) Crocin reduces the inflammation response in rheumatoid arthritis. Biosci Biotechnol Biochem 81:891–898
Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291–295
Amor S, Woodroofe MN (2014) Innate and adaptive immune responses in neurodegeneration and repair. Immunology 141:287–291
Pashirzad M, Shafiee M, Avan A, Ryzhikov M, Fiuji H, Bahreyni A et al (2019) Therapeutic potency of crocin in the treatment of inflammatory diseases: current status and perspective. J Cell Physiol 234(9):14601–14611
Li S, Liu X, Lei J, Yang J, Tian P, Gao Y (2017) Crocin protects podocytes against oxidative stress and inflammation induced by high glucose through inhibition of NF-κB. Cell Physiol Biochem 42:1481–1492
Mazumder AG, Sharma P, Patial V, Singh D (2017) Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-κB activation. Basic Clin Pharmacol Toxicol 120:426–433
Lv B, Chen T, Xu Z, Huo F, Wei Y, Yang X (2016) Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int J Mol Med 37:225–232
Zhang G-F, Zhang Y, Zhao G (2015) Crocin protects PC12 cells against MPP+-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem Int 89:101–110
Okouchi M, Ekshyyan O, Maracine M, Aw TY (2007) Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal 9:1059–1096
Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ et al (2000) Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci 20:7994–8004
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Cho D-H, Nakamura T, Lipton SA (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci 67:3435–3447
Artal-Sanz M, Tavernarakis N (2005) Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett 579:3287–3296
Turner C, Schapira AH (2001) Mitochondrial dysfunction in neurodegenerative disorders and ageing. In: Neuropathology and genetics of dementia. Springer, Boston, pp 229–251
Conrad M, Schick J, Angeli JPF (2013) Glutathione and thioredoxin dependent systems in neurodegenerative disease: what can be learned from reverse genetics in mice. Neurochem Int 62:738–749
Eldadah BA, Faden AI (2000) Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma 17:811–829
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885
Mattson MP (2006) Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal 8:1997–2006
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochimica et Biophysica Acta (BBA) 1644:189–203
Shacka JJ, Roth KA (2005) Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr Drug Targets-CNS Neurol Disord 4:25–39
Luo X-G, Ding J-Q, Chen S-D (2010) Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegen 5:12
Breydo L, Redington J, Uversky V (2017) Effects of intrinsic and extrinsic factors on aggregation of physiologically important intrinsically disordered proteins. Int Rev Cell Mol Biol 329:145–185
Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ et al (2004) Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41:549–561
Yang J-L, Weissman L, Bohr VA, Mattson MP (2008) Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair 7:1110–1120
Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ et al (2006) The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature 443:713
Brasnjevic I, Hof PR, Steinbusch HW, Schmitz C (2008) Accumulation of nuclear DNA damage or neuron loss: molecular basis for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases. DNA Repair 7:1087–1097
Wright AF, Jacobson SG, Cideciyan AV, Roman AJ, Shu X, Vlachantoni D et al (2004) Lifespan and mitochondrial control of neurodegeneration. Nat Genet 36:1153
Beurel E, Jope RS (2006) The paradoxical pro-and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79:173–189
Felderhoff-Mueser U, Sifringer M, Pesditschek S, Kuckuck H, Moysich A, Bittigau P et al (2002) Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis 11:231–245
Rao RV, Bredesen DE (2004) Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol 16:653–662
Soane L, Kahraman S, Kristian T, Fiskum G (2007) Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J Neurosci Res 85:3407–3415
Tabner BJ, Turnbull S, El-Aganf O, Allsop D (2001) Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr Top Med Chem 1:507–517
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167
Mytilineou C, Kramer BC, Yabut JA (2002) Glutathione depletion and oxidative stress. Parkinsonism Relat Disord 8:385–387
Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U (2002) Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 22:1–7
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm 5:45
Blasko I, Ransmayr G, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B (2001) Does IFNγ play a role in neurodegeneration? J Neuroimmunol 116:1–4
Huang Z, Xu J, Huang X, Sun G, Jiang R, Wu H et al (2019) Crocin induces anti-ischemia in middle cerebral artery occlusion rats and inhibits autophagy by regulating the mammalian target of rapamycin. Eur J Pharmacol 857:172424
Mozaffari S, Yasuj SR, Motaghinejad M, Motevalian M, Kheiri R (2019) Crocin acting as a neuroprotective agent against methamphetamine-induced neurodegeneration via CREB-BDNF signaling pathway. Iran J Pharm Res 18:745
Shafahi M, Vaezi G, Shajiee H, Sharafi S, Khaksari M (2018) Crocin inhibits apoptosis and astrogliosis of hippocampus neurons against methamphetamine neurotoxicity via antioxidant and anti-inflammatory mechanisms. Neurochem Res 43:2252–2259
Hosseinzadeh H, Abootorabi A, Sadeghnia HR (2008) Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate–induced DNA damage in mice organs. DNA Cell Biol 27:657–664
Abe K, Saito H (2000) Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res 14:149–152
Eteqadi M, Nasehi M, Hesami TS (2017) Effect of crocin on mitochondrial biogenesis in the striatum of cholestatic male wistar rats. 1st International Congress on Biomedicine (ICB), Tehran
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kermanshahi, S., Ghanavati, G., Abbasi-Mesrabadi, M. et al. Novel Neuroprotective Potential of Crocin in Neurodegenerative Disorders: An Illustrated Mechanistic Review. Neurochem Res 45, 2573–2585 (2020). https://doi.org/10.1007/s11064-020-03134-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03134-8