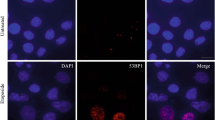
Abstract
Telomeres, the specialized nucleoproteic complexes localized at the physical ends of linear eukaryotic chromosomes, play a fundamental role in maintaining chromosomal stability and integrity, being one of the leading guardians of genome stability. In recent years, the identification and analysis of chromosomal aberrations involving telomeres has proven to be a unique tool to evaluate misrepaired and unrepaired chromosome damage in mammalian cells. Telomere instability constitutes an important source of genomic instability, a phenomenon characteristic of cancer cells, and also common in cells exposed to chemical or physical mutagens which induce chromosomal aberrations by producing chromosome breakage (clastogens). In the present review, we will focus on the chromosomal aberrations involving telomeres and their importance to determine the clastogen-induced genomic instability present in mammalian cells.



Similar content being viewed by others
Abbreviations
- BFB:
-
Breakage-fusion-bridge
- CF:
-
Compound fragment
- DDR:
-
DNA damage response
- DSB:
-
Double-strand break/s
- EBV:
-
Epstein-Barr Virus
- HR:
-
Homologous recombination
- IC:
-
Incomplete chromosome
- ICE:
-
Incomplete chromosome elements
- IF:
-
Interstitial fragment
- NHEJ:
-
Non-homologous end joining
- ssDNA:
-
Single-stranded DNA
- SSB:
-
Single-strand break/s
- TF:
-
Terminal fragment
References
Ahmed W, Lingner J (2018) Impact of oxidative stress on telomere biology. Differentiation 99:21–27
Al-Wahiby S, Slijepcevic P (2005) Chromosomal abnormalities in BRCA1 deficient human and mouse cell lines. Cytogenet Genome Res 109:491–496
Arnoult N, Karlseder J (2015) Complex interactions between the DNA-damage response and mammalian telomeres. Nat Struct Mol Biol 22:859–866
Artandi SE, DePinho RA (2010) Telomeres and telomerase in cancer. Carcinogenesis 31:9–18
Ayouaz A, Raynaud C, Heride C, Revaud D, Sabatier L (2008) Telomeres: hallmarks of radiosensitivity. Biochimie 90:60–72
Azzalin CM, Lingner J (2008) Telomeres: the silence is broken. Cell Cycle 7:1161–1165
Bailey SM, Cornforth MN, Ullrich RL, Goodwin EH (2004a) Dysfunctional mammalian telomeres join with DNA double-strand breaks. DNA Repair 3:349–357
Bailey SM, Goodwin EH, Cornforth MN (2004b) Strand-specific fluorescence in situ hybridization: the CO-FISH family. Cytogenet Genome Res 107:14–17
Balajee AS, Hande MP (2018) History and evolution of cytogenetic techniques: current and future applications in basic and clinical research. Mutat Res 836:3–12
Barnes RP, Fouquerel E, Opresko PL (2019) The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177:37–45
Bejarano L, Bosso G, Louzame J, Serrano R, Gómez-Casero E, Martínez-Torrecuadrada J, Martínez S, Blanco-Aparicio C, Pastor J, Blasco MA (2019) Multiple cancer pathways regulate telomere protection. EMBO Mol Med 11:e10292
Benkhaled L, Barrios L, Mestres M, Caballin MR, Ribas M, Barquinero JF (2006) Analysis of gamma-rays induced chromosome aberrations: a fingerprint evaluation with a combination of pan-centromeric and pan-telomeric probes. Int J Radiat Biol 82:869–875
Benkhaled L, Xunclá M, Caballín MR, Barrios L, Barquinero JF (2008) Induction of complete and incomplete chromosome aberrations by bleomycin in human lymphocytes. Mutat Res 637:134–141
Berardinelli F, Antoccia A, Cherubini R, De Nadal V, Gerardi S, Cirrone GA, Tanzarella C, Sgura A (2010) Transient activation of the ALT pathway in human primary fibroblasts exposed to high-LET radiation. Radiat Res 174:539–549
Berardinelli F, Antoccia A, Buonsante R, Gerardi S, Cherubini R, De Nadal V, Tanzarella C, Sgura A (2013) The role of telomere length modulation in delayed chromosome instability induced by ionizing radiation in human primary fibroblasts. Environ Mol Mutagen 54:172–179
Berardinelli F, Sgura A, Di Masi A, Leone S, Cirrone GA, Romano F, Tanzarella C, Antoccia A (2014) Radiation-induced telomere length variations in normal and in Nijmegen Breakage Syndrome cells. Int J Radiat Biol 90:45–52
Berardinelli F, Coluzzi E, Sgura A, Antoccia A (2017) Targeting telomerase and telomeres to enhance ionizing radiation effects in in vitro and in vivo cancer models. Mutat Res 773:204–219
Bettin N, Pegorar CO, Cusanelli E (2019) The emerging roles of TERRA in telomere maintenance and genome instability. Cells 8:246
Bhargava R, Fischer M, O’Sullivan RJ (2020) Genome rearrangements associated with aberrant telomere maintenance. Curr Opin Genet Dev 60:31–40
Blackburn EH (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579:859–862
Boei JJWA, Natarajan AT (1998) Combined use of chromosome painting and telomere detection to analyse radiation induced chromosomal aberrations in mouse splenocytes. Int J Radiat Biol 73:125–133
Boei JJWA, Vermeulen S, Fomina J, Natarajan AT (1998) Detection of incomplete exchanges and interstitial fragments in X-irradiated human lymphocytes using a telomeric PNA probe. Int J Radiat Biol 73:599–603
Boei JJWA, Vermeulen S, Natarajan AT (2000) Analysis of radiation-induced chromosomal aberrations using telomeric and centromeric PNA probes. Int J Radiat Biol 76:163–167
Boei JJWA, Vermeulen S, Mullenders LHF, Natarajan AT (2001) Impact of radiation quality on the spectrum of induced chromosome exchange aberrations. Int J Radiat Biol 77:847–857
Bolzán AD (2009) Cytogenetic evaluation of telomere dysfunction: chromosomal aberrations involving telomeres and interstitial telomeric sequences. In: Mancini L (ed) Telomeres: function, shortening and lengthening. Nova Science Publishers Inc., New York, pp 133–185
Bolzán AD (2012) Chromosomal aberrations involving telomeres and interstitial telomeric sequences. Mutagenesis 27:1–15
Bolzán AD (2016) Telomere instability induced by anticancer drugs in mammalian cells. In: Larramendy M (ed) Telomere: a complex end of a chromosome. InTech Open Access Publisher, Rijeka, pp 119–141
Bolzán AD, Bianchi MS (2004a) Detection of incomplete chromosome elements and interstitial fragments induced by bleomycin in hamster cells using a telomeric PNA probe. Mutat Res 554:1–8
Bolzán AD, Bianchi MS (2004b) Analysis of streptonigrin-induced incomplete chromosome elements and interstitial fragments in Chinese hamster cells using a telomeric PNA probe. Environ Mol Mutagen 44:277–282
Bolzán AD, Bianchi MS (2005) Analysis of streptozotocin-induced incomplete chromosome elements and excess acentric fragments in Chinese hamster cells using a telomeric PNA probe. Mutat Res 570:237–244
Bolzán AD, Bianchi MS (2006) Telomeres, interstitial telomeric repeat sequences, and chromosomal aberrations. Mutat Res 612:189–214
Bouffler SD, Morgan WF, Pandita TK, Slijepcevic P (1996) The involvement of telomeric sequences in chromosomal aberrations. Mutat Res 366:129–135
Brault ME, Autexier C (2011) Telomeric recombination induced by dysfunctional telomeres. Mol Biol Cell 22:179–188
Caradonna F (2015) Nucleoplasmic bridges and acrocentric chromosome associations as early markers of exposure to low levels of ionising radiation in occupationally exposed hospital workers. Mutagenesis 30:269–275
Chan SR, Blackburn EH (2004) Telomeres and telomerase. Philos Trans R Soc Lond Ser B Biol Sci 359:109–121
Chavez A, Tsou AM, Johnson FB (2009) Telomeres do the (un)twist: helicase actions at chromosome termini. Biochim Biophys Acta 1792:329–340
Cherdyntseva V, Gagos S (2020) Chromosome extremities under the microscopy lens: molecular cytogenetics in telomere research. Curr Opin Genet Dev 60:69–76
Cheung AL, Deng W (2008) Telomere dysfunction, genome instability and cancer. Front Biosci 13:2075–2090
Chiodi I, Belgiovine C, Mondello C (2010) Telomerase and telomeric proteins: a life beyond telomeres. In: Gagnon AN (ed) Telomerase: composition, functions and clinical implications. Nova Science, New York, pp 35–58
Cicconi A, Chang S (2020) Shelterin and the replisome: at the intersection of telomere repair and replication. Curr Opin Genet Dev 60:77–84
Cleal K, Baird DM (2020) Catastrophic endgames: emerging mechanisms of telomere-driven genomic instability. Trends Genet 36:347–359
Cusanelli E, Chartrand P (2015) Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity. Front Genet 6:1–9
Denchi EL (2009) Give me a break: how telomeres suppress the DNA damage response. DNA Repair 8:1118–1126
Dewhurst SM (2020) Chromothripsis and telomere crisis: engines of genome instability. Curr Opin Genet Dev 60:41–47
Díaz Flaqué MC, Bianchi MS, Bolzán AD (2006) A comparative analysis of bleomycin-induced incomplete chromosome elements in two mammalian cell lines using a telomeric PNA probe. Environ Mol Mutagen 47:674–681
Doksani Y (2019) The response to DNA damage at telomeric repeats and its consequences for telomere function. Genes 10:318
Draskovic I, Londono-Vallejo A (2014) Telomere recombination and the ALT pathway: a therapeutic perspective for cancer. Curr Pharm Des 20:6466–6471
Durante M, George K, Cucinotta FA (2006) Chromosomes lacking telomeres are present in the progeny of human lymphocytes exposed to heavy ions. Radiat Res 165:51–58
Feijoo P, Dominguez D, Tusell L, Genesca A (2014) Telomere-dependent genomic integrity: evolution of the fusion-bridge-breakage cycle concept. Curr Pharm Des 20:6375–6385
Fimon P, Wong HP, Silver AR, Slijepcevic P, Bouffler SD (2001) Long but dysfunctional telomeres correlated with chromosomal radiosensitivity in a mouse AML cell line. Int J Radiat Biol 77:1151–1162
Fomina J, Darroudi F, Boei JJWA, Natarajan AT (2000) Discrimination between complete and incomplete chromosome exchanges in X-irradiated human lymphocytes using FISH with pan-centromeric and chromosome specific DNA probes in combination with telomeric PNA probe. Int J Radiat Biol 76:807–813
Fomina J, Darroudi F, Natarajan AT (2001) Accurate detection of true incomplete exchanges in human lymphocytes exposed to neutron radiation using chromosome painting in combination with a telomeric PNA probe. Int J Radiat Biol 77:1175–1183
Fomina J, Darroudi F, Natarajan AT (2002) Incomplete chromosome exchanges are not fingerprints of high LET neutrons. Radiat Prot Dosim 99:215–216
Hoang SM, O’Sullivan RJ (2020) Alternative lengthening of telomeres: building bridges to connect chromosome ends. Trends Cancer 6:247–260
Hyeon Joo O, Hande MP, Lansdorp PM, Natarajan AT (1998) Induction of telomerase activity and chromosome aberrations in human tumour cell lines following X-irradiation. Mutat Res 401:121–131
Kamranvar SA, Chen X, Masucci MG (2013) Telomere dysfunction and activation of alternative lengthening of telomeres in B-lymphocytes infected by Epstein-Barr virus. Oncogene 32:5522–5530
Lacoste S, Wiechec E, dos Santos Silva AG, Guffei A, Williams G, Lowbeer M, Benedek K, Henriksson M, Klein G, Mai S (2010) Chromosomal rearrangements after ex vivo Epstein-Barr virus (EBV) infection of human B cells. Oncogene 29:503–515
Liu H, Xie Y, Zhang Z, Mao P, Liu J, Ma W, Zhao Y (2018) Telomeric recombination induced by DNA damage results in telomere extension and length heterogeneity. Neoplasia 20:905–916
Liu J, Wang L, Wang Z, Liu J-P (2019) Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells 8:54
Luijten MNH, Lee JXT, Crasta KC (2018) Mutational game changer: chromothripsis and its emerging relevance to cancer. Mutat Res 777:29–51
Luke B, Lingner J (2009) TERRA: telomeric repeat-containing RNA. EMBO J 28:2503–2510
M’Kacher R, Maalouf EE, Ricoul M, Heidingsfelder L, Laplagne E, Cuceu C, Hempel WM, Colicchio B, Dieterlen A, Sabatier L (2014) New tool for biological dosimetry: reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat Res 770:45–53
M’Kacher R, Colicchio B, Borie C, Junker S, Marquet V, Heidingsfelder L, Soehnlen K, Najar W, Hempel WM, Oudrhiri N, Wilhelm-Murer N, Miguet M, Arnoux M, Ferrapie C, Kerbrat W, Plesch A, Dieterlen A, Girinsky T, Voisin P, Deschenes G, Tabet AC, Yardin C, Bennaceur-Griscelli A, Fenech M, Carde P, Jeandidier E (2020) Telomere and centromere staining followed by M-FISH improves diagnosis of chromosomal instability and its clinical utility. Genes 11:475
Maciejowski J, de Lange T (2017) Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 18:175–186
Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T (2015) Chromothripsis and kataegis induced by telomere crisis. Cell 163:1641–1654
McHugh MM, Gawron LS, Matsui S, Beerman TA (2005) The antitumor enediyne C-1027 alters cell cycle progression and induces chromosomal aberrations and telomere dysfunction. Cancer Res 65:5344–5351
McKenna MJ, Robinson E, Goodwin EH, Cornforth MN, Bailey SM (2017) Telomeres and NextGen CO-FISH: Directional Genomic Hybridization (Telo-dGH™). Methods Mol Biol 1587:103–112
Mestres M, Caballín MR, Schmid E, Stephan G, Sachs R, Barrios L, Barquinero JF (2004) Analysis of α-particle induced chromosome aberrations in human lymphocytes, using pan-centromeric and pan-telomeric probes. Int J Radiat Biol 80:737–744
Mestres M, Benkhaled L, Caballín MR, Barrios L, Ribas M, Barquinero JF (2011) Induction of incomplete and complex chromosome aberrations by 30 kVp X rays. Radiat Res 175:201–207
Multani AS, Li C, Ozen M, Yadav M, Yu DF, Wallace S, Pathak S (1997) Paclitaxel and water-soluble poly (L-glutamic acid)-paclitaxel, induce direct chromosomal abnormalities and cell death in a murine metastatic melanoma cell line. Anticancer Res 17:4269–4274
Multani AS, Li C, Ozen M, Imam AS, Wallace S, Pathak S (1999) Cell-killing by paclitaxel in a metastatic murine melanoma cell line is mediated by extensive telomere erosion with no decrease in telomerase activity. Oncol Rep 6:39–44
Murnane JP (2012) Telomere dysfunction and chromosome instability. Mutat Res 730:28–36
O’Sullivan RJ, Karlseder J (2010) Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11:171–181
Ojima M, Hamano H, Suzuki M, Suzuki K, Kodama S, Watanabe M (2004) Delayed induction of telomere instability in normal human fibroblast cells by ionizing radiation. J Radiat Res 45:105–110
Paeschke K, McDonald KR, Zakian VA (2010) Telomeres: structures in need of unwinding. FEBS Lett 584:3760–3772
Pampalona J, Soler D, Genescà A, Tusell L (2010) Whole chromosome loss is promoted by telomere dysfunction in primary cells. Genes Chromosom Cancer 49:368–378
Paviolo NS, Quiroga IY, Castrogiovanni DC, Bianchi MS, Bolzán AD (2012) Telomere instability is present in the progeny of mammalian cells exposed to bleomycin. Mutat Res 734:5–11
Paviolo NS, Castrogiovanni DC, Bolzán AD (2014) The radiomimetic compound streptonigrin induces persistent telomere dysfunction in mammalian cells. Mutat Res 760:16–23
Paviolo NS, Santiñaque FF, Castrogiovanni DC, Folle GA, Bolzán AD (2015) The methylating agent streptozotocin induces persistent telomere dysfunction in mammalian cells. Mutat Res 794:17–24
Pellestor F (2019) Chromoanagenesis: cataclysms behind complex chromosomal rearrangements. Mol Cytogenet 12:6
Pellestor F, Paulasova P (2004) The peptide nucleic acids (PNAs): introduction to a new class of probes for chromosomal investigation. Chromosoma 112:375–380
Poon SS, Martens UM, Ward RK, Lansdorp PM (1999) Telomere length measurements using digital fluorescence microscopy. Cytometry 36:267–278
Popuri V, Hsu J, Khadka P, Horvath K, Liu Y, Croteau DL, Bohr VA (2014) Human RECQL1 participates in telomere maintenance. Nucleic Acids Res 42:5671–5688
Pottier G, Viau M, Ricoul M, Shim G, Bellamy M, Cuceu HWM, Sabatier L (2013) Lead exposure induces telomere instability in human cells. PLoS One 8:e67501
Pujol-Canadell M, Puig R, Armengol G, Barrios L, Barquinero JF (2020) Chromosomal aberration dynamics through the cell cycle. DNA Repair 89:102838
Rao CV, Yamada HY, Yao Y, Dai W (2009) Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice. Carcinogenesis 30:1469–1474
Ricoul M, Gnana Sekaran TS, Brochard P, Herate C, Sabatier L (2019) γ-H2AX foci persistence at chromosome break suggests slow and faithful repair phases restoring chromosome integrity. Cancers 11:1397
Rodríguez P, Barquinero JF, Duran A, Caballín MR, Ribas M, Barrios L (2009) Cells bearing chromosome aberrations lacking one telomere are selectively blocked at the G2/M checkpoint. Mutat Res 670:53–58
Romaniuk A, Paszel-Jaworska A, Totoń E, Lisiak N, Holysz H, Królak A, Grodecka-Gazdecka S, Rubis B (2019) The non-canonical functions of telomerase: to turn off or not to turn off. Mol Biol Rep 46:1401–1411
Schmutz I, de Lange T (2016) Shelterin. Curr Biol 26:R397–R399
Sharma GG, Hall EJ, Dhar S, Gupta A, Rao PH, Pandita TK (2003) Telomere stability correlates with longevity of human beings exposed to ionizing radiations. Oncol Rep 10:1733–1736
Shay JW (2018) Telomeres and aging. Curr Op Cell Biol 52:1–7
Shay JW, Wright WE (2019) Telomeres and telomerase: three decades of progress. Nat Rev Genet 20:299–309
Shim G, Ricoul M, Hempel WM, Azzam EI, Sabatier L (2014) Crosstalk between telomere maintenance and radiation effects: a key player in the process of radiation-induced carcinogenesis. Mutat Res 760:1–17
Storchová Z, Kloosterman WP (2016) The genomic characteristics and cellular origin of chromothripsis. Curr Opin Cell Biol 40:106–113
Stroik S, Hendrickson EA (2020) Telomere fusions and translocations: a bridge too far? Curr Opin Genet Dev 60:85–91
Undarmaa B, Kodama S, Suzuki K, Niwa O, Watanabe M (2004) X-ray-induced telomeric instability in Atm-deficient mouse cells. Biochem Biophys Res Commun 315:51–58
Uringa EJ, Lisaingo K, Pickett HA, Brind’Armour J, Rohde JH, Zelensky A, Essers J, Lansdorp PM (2012) RTEL1 contributes to DNA replication and repair and telomere maintenance. Mol Biol Cell 23:2782–2792
Venkatesan S, Natarajan AT, Hande MP (2015) Chromosomal instability-mechanisms and consequences. Mutat Res 793:176–184
Volleth M, Zenker M, Joksic I, Liehr T (2020) Long-term culture of EBV-induced human lymphoblastoid cell lines reveals chromosomal instability. J Histochem Cytochem 68:239–251
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wu H, George K, Yang TC (1998) Estimate of true incomplete exchanges using fluorescence in situ hybridization with telomere probes. Int J Radiat Biol 73:521–527
Wu H, George K, Yang TC (1999) Estimate of the frequency of true incomplete exchanges in human lymphocytes exposed to 1 GeV/u Fe ions in vitro. Int J Radiat Biol 75:593–599
Yao Y, Dai W (2014) Genomic instability and cancer. J Carcinog Mutagen 5:1000165
Zeegers D, Venkatesan S, Koh SW, Low GK, Srivastava P, Sundaram N, Sethu S, Banerjee B, Jayapal M, Belyakov O, Baskar R, Balajee AS, Hande MP (2017) Biomarkers of ionizing radiation exposure: a multiparametric approach. Genome Integr 8:6
Zhang J-M, Zou L (2020) Alternative lengthening of telomeres: from molecular mechanisms to therapeutic outlooks. Cell Biosci 10:30
Funding
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP No. 0182), the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CICPBA), and the University of La Plata (UNLP) of Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Responsible Editor: Beth Sullivan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bolzán, A.D. Using telomeric chromosomal aberrations to evaluate clastogen-induced genomic instability in mammalian cells. Chromosome Res 28, 259–276 (2020). https://doi.org/10.1007/s10577-020-09641-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-020-09641-2