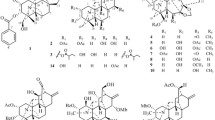
A new indole alkaloid (1), together with eight known indole alkaloid derivatives (2–9), was isolated from Hosta plantaginea for the first time. The structures of compounds 1–9 were elucidated on the basis of comprehensive spectroscopic analyses and references. Compounds 1–9 were evaluated for their inhibition of steroid 5α-reductase activity in vitro. Among them, compounds 1 and 2 showed important inhibition of steroid 5α-reductase activities.

Similar content being viewed by others
References
J. Feng, L. F. Hu, and Y. Tang, Pharm. Clin. Chin. Mater. Med., 59, 8 (2017).
J. W. He, L. Yang, and G. Y. Zhong, Chin. Trad. Herb. Drugs, 47, 4295 (2016).
J. Y. Qu, M. Y. Wang, C. M. Wang, G. Y. Zhong, and X. B. Li, Chin. Trad. Herb. Drugs, 42, 217 (2011).
J. Q. Liu, C. F. Wang, M. H. Qiu, and W. X. Hu, Chin. Trad. Herb. Drugs, 41, 520 (2010).
X. H. Bao, Q. H. Wang, B. Y. Q. E. Bao, J. J. Han, and W. L. J. Ao, Chem. Nat. Compd., 53, 614 (2017).
Y. Xin and Y. H. Bai, Chin. Trad. Pat. Med., 37, 653 (2015).
R. R. Wei, Q. G. Ma, G. Y. Zhong, J. W. He, and Z. P. Sang, J. Ethnopharmacol., 253, 112685 (2020).
Y. H. Wang, Z. K. Zhang, F. M. Yang, Q. Y. Sun, H. P. He, Y. T. Di, S. Z. Mu, Y. Lu, Y. Chang, Q. T. Zheng, M. Ding, J. H. Dong, and X. J. Hao, J. Nat. Prod., 70, 1458 (2007).
H. X. Xie, J. H. Zhang, H. G. Zhang, and P. F. Xue, Chin. Pharm. J., 44, 733 (2009).
J. H. Zhang, H. G. Xie, P. F. Xue, H. G. Zhang, X. Y. Liu, and M. Baijie, Chin. Pharm. J., 45, 335 (2010).
K. B. Wang, C. M. Yuan, C. M. Xue, D. H. Li, Y. K. Jing, H. P. He, X. J. Hao, Y. T. Di, Z. L. Li, and H. M. Hua, RSC Adv., 4, 53725 (2014).
C. Y. Wang, J. D. Hao, X. Y. Ning, J. S. Wu, D. L. Zhao, C. J. Kong, C. L. Shao, and C. Y. Wang, RSC Adv., 8, 4348 (2018).
C. Wu, L. J. He, X. Yi, J. Qin, Y. L. Li, Y. B. Zhang, and G. C. Wang, J. Nat. Med., 73, 667 (2019).
J. B. Bremner, W. Sengpracha, I. Southwell, C. Bourke, B. W. Skelton, and A. H. White, Aust. J. Chem., 57, 273 (2004).
G. Abbiati, E. M. Beccalli, A. Marchesini, and E. Rossi, Synthesis, 16, 2477 (2001).
Y. Hirasawa, X. Dai, J. Deguchi, S. Hatano, T. Sasaki, R. Ohtsuka, A. E. Nugroho, T. Kaneda, and H. Morita, J. Nat. Med., 73, 627 (2019).
M. Safratova, A. Hostalkova, D. Hulcova, K. Breiterova, V. Hrabcova, M. Machado, D. Fontinha, M. Prudencio, J. Kunes, J. Chlebek, D. Jun, M. Hrabinova, L. Novakova, R. Havelek, M. Seifrtova, L. Opletal, and L. Cahlikova, Arch. Pharm. Res., 41, 208 (2018).
Q. G. Ma, R. R. Wei, M. Yang, X. Y. Huang, G. Y. Zhong, Z. P. Sang, J. H. Dong, J. C. Shu, J. Q. Liu, R. Zhang, J. B. Yang, A. G. Wang, T. F. Ji, and Y. L. Su, Bioorg. Chem., 86, 159 (2019).
J. Xin, W. S. Tian, Y. Y. Sun, and Z. H. Tu, Chin. J. Pharmacol. Toxicol., 17, 395 (2003).
Acknowledgment
This research program is financially supported by the National Natural Science Foundation of China (81803843), the Scientific Research Project of Jiangxi Administration of Traditional Chinese Medicine (2019A002, 2019A018), the Standard Revision Research Project of National Pharmacopoeia Committee (2018Z090), the Science and Technology Project of Jiangxi Provincial Department of Education (GJJ180688, GJJ180662), the Science and Technology Project of Jiangxi Health Commission (20195650, 20195648), the Scientific Research Project of First-class Discipline of Chinese Materia Medica of Jiangxi University of TCM (JXSYLXK-ZHYAO027, JXSYLXK-ZHYAO032), and the Doctoral Research Initiation Fund Project of Jiangxi University of TCM (2018BSZR010, 2018BSZR007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2020, pp. 760–762.
Rights and permissions
About this article
Cite this article
Wei, R., Ma, Q. Indole Alkaloids from Hosta plantaginea and Inhibition of Steroid 5α-Reductase Activities In Vitro. Chem Nat Compd 56, 888–891 (2020). https://doi.org/10.1007/s10600-020-03176-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03176-y