Abstract
Background
Numerous phylogenetic markers have been tested over a period of time for delineating evolutionary history of haemoflagellate—Trypanosoma evansi.
Purpose
To find out the associative genetic diversity, within the various isolates of T. evansi across the globe, based on RoTat 1.2 VSG gene.
Methods
A total of 5 equine isolates of T. evansi from Northern India were characterized. PCR products were sequenced and sequences were compared with available sequences across India and world. Phylogenetic tree was constructed based on maximum parsimony (MP) method with the tree-bisection-regrafting (TBR) algorithm.
Results
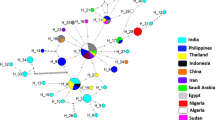
Indian isolates formed multiple clades with two haplotypes. The present isolates showed 99.49–100.00% nucleotide homology within themselves. On broader line, Indian isolates were found to be closer to Egyptian isolates than the African counterparts. Few of the Indian isolates showed marked resemblance with a particular Egyptian isolate than with their Indian counter parts. Another remarkable finding is the close association of equine isolates from India with other equine isolates and their clear divergence from isolates of T. evansi affecting other hosts from India and abroad.
Conclusion
Vast genetic divergence was seen between the isolates suggesting of multiple distinct lineages of T. evansi amongst the Indian livestock. Interestingly, variations in sequences were seen based on the host range of isolates. The findings are very important from molecular evolutionary point of view.



Similar content being viewed by others
References
Sudan V, Jaiswal AK, Verma AK (2017) Trypanosomosis of wild animals with emphasis on Indian scenario. Vet Parasitol Reg Stud Rep 10:25–28. https://doi.org/10.1016/j.vprsr.2017.07.003
Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S (2013) Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts and pathogenic effects. BioMed Res Int. https://doi.org/10.1155/2013/194176
Pandey V, Nigam R, Jiaswal AK, Sudan V, Singh RK, Yadav PK (2015) Haemato biochemical and oxidative status of buffaloes naturally infected with Trypanosoma evansi. Vet Parasitol 212:118–222. https://doi.org/10.1016/j.vetpar.2015.07.025
Jaiswal A, Sudan V, Neha VAK (2015) Insight into trypanosomiasis in animals: various approaches for its diagnosis, treatment and control: a review. Asian J Anim Sci 9(5):172–186. https://doi.org/10.3923/ajas.2015.172.186
Barry JD, McCullogh R (2001) Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol 49:1–70. https://doi.org/10.1016/S0065-308X(01)49037-3
Verloo D, Magnus E, Buscher P (2001) General expression of RoTat 1.2 variable antigen type in Trypanosoma evansi isolates from different origin. Vet Parasitol 97:183–189. https://doi.org/10.1016/s0304-4017(01)00412-5
Zhang ZQ, Baltz T (1994) Identification of Trypanosoma evansi. Trypanosoma equiperdum and Trypanosoma brucei brucei using repetitive DNA probes. Vet Parasitol 53:197–208. https://doi.org/10.1016/0304-4017(94)90183-x
Sengupta PP, Balumahendiran M, Balamurugan V, Rudramurthy GR, Prabhudas K (2012) Expressed truncated N-terminal variable surface glycoprotein (VSG) of Trypanosoma evansi in E. coli exhibits immuno-reactivity. Vet Parasitol 187:1–8. https://doi.org/10.1016/j.vetpar.2012.01.012
Sengupta PP, Balumahendiran M, Suryanarayana VVS, Raghavendra AG, Shome BR, Ganjendragad MR, Prabhudas K (2010) PCR-based diagnosis of surra-targeting VSG gene: experimental studies in small laboratory rodents and buffalo. Vet Parasitol 171:22–31. https://doi.org/10.1016/j.vetpar.2010.03.011
Devi A, Shanker D, Sudan V, Chaudhaury M (2017) PCR-based diagnosis of surra in equines targeting RoTat 1.2 VSG gene. J Vet Parasitol 31(2):74–78
Devi A, Shanker D, Sudan V, Singh JAK, Chaudhaury AM (2018) Phylogenetic studies on RoTat 1.2 VSG of Trypanosoma evansi isolate from semi arid India. Indian J Anim Sci 88(2):150–152
Devi A, Shanker D, Sudan V, Jaiswal AK, Singh A (2017) Molecular characterization and phylogenetic sequence analysis of unique conserved portion of VSG of Trypanosoma evansi. India J Anim Sci 87(8):974–976
Sudan V, Jaiswal AK, Shanker D, Verma AK (2017) First report of molecular characterization and phylogenetic analysis of RoTat 1.2 VSG of Trypanosoma evansi from equine isolate. Trop Anim Health Prod 49(8):1793–1796. https://doi.org/10.1007/s11250-017-1384-7
Patel G, Shanker D, Jaiswal AK, Sudan V, Verma SK (2013) Prevalence and seasonal variation in ixodid ticks on cattle of Mathura district, Uttar Pradesh. J Parasit Diseases 37(2):173–176. https://doi.org/10.1007/s12639-012-0154-8
Gaur R, Sudan V, Jaiswal AK, Singh A, Shanker D (2017) Classico-molecular targeting of oligopeptidase B, cysteine protease and variable surface glycoprotein (VSG) genes of Trypanosoma evansi. J Parasit Diseases 41(1):51–54. https://doi.org/10.1007/s12639-016-0748-7
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Sudan V, Shanker D, Jaiswal AK, Singh A, Parashar R (2017) Molecular characterization of serine oligopeptidase B gene of Trypanosoma evansi equine isolate from semi-arid India and its phylogenetic analysis with other trypanosomatids. J Vet Parasitol 31(1):30–36
Sudan V, Jaiswal AK, Shanker D (2018) Heat shock protein 70 of Trypanosoma evansi is phylogenetically closer to salivaria than stercoraria homologs. Comp Clin Pathol 27:245–248. https://doi.org/10.1007/s00580-017-2570-8
Li SQ, Fung MC, Reid SA, Inoue N, Lun ZR (2007) Immunizationwith recombinant beta-tubulin from Trypanosoma evansi induced protein T. evansi, T. equiperdum and T.b. brucei infection in mice. Parasite Immunol 29:191–199. https://doi.org/10.1111/j.1365-3024.2006.00933.x
Li SQ, Yang WB, Lun ZR, Ma LJ, Xi SM, Chen QL, Song XW, Kang J, Yang LZ (2009) Immunization with recombinant actin from Trypanosoma evansi induces protective immunity against T. evansi, T. equiperdum and T.b. brucei infection. Parasitol Res 104:429–435. https://doi.org/10.1007/s00436-008-1216-9
Tran T, Buscher P, Vandenbussche G, Wyns L, Messens J, Greve HD (2008) Heterologous expression, purification and characterization of the extra cellular domain of trypanosome invariant surface glycoprotein ISG75. J Biotechnol 135:247–254. https://doi.org/10.1016/j.jbiotec.2008.04.012
Allen TE, Ullman B (1993) Cloning and expression of the hypoxanthine-guanine phosphoribosyltransferase gene from Trypanosoma brucei. Nucleic Acids Res 21:5431–5438. https://doi.org/10.1093/nar/21.23.5431
Ngaira JM, Njagi EN, Ngeranwa JJ, Olembo NK (2004) PCR amplification of RoTat 1.2 VSG gene in Trypanosoma evansi isolates in Kenya. Vet Parasitol 120:23–33. https://doi.org/10.1016/j.vetpar.2003.12.007
Salim B, Bakheit MA, Kamau J, Nakamura I, Sugimoto C (2011) Molecular epidemiology of camel trypanosomiasis based on ITS1rDNA and RoTat 1.2 VSG gene in the Sudan. Parasite Vectors 4:31. https://doi.org/10.1186/1756-3305-4-31
Sudan V, Tewari AK, Singh H (2015) A native whole cell lysate antigen (WCLA) based ELISA for the sero-detection of surra in Indian cattle. Indian J Anim Sci 85(6):601–603
Acknowledgements
The authors are highly thankful to the Director Research, Dean CoVSc and Vice Chancellor, DUVASU, for the facilities provided. The authors also want to acknowledge the various funding agencies like Indian Council of Agricultural Research (ICAR) and Rashtriya Krishi Vikas Yojana (RKVY) for sanctioning various projects to the University for the procurement of instruments to carry out such work. The authors also acknowledge Incharge, Central Instrumentation Facility (CIF) for permitting the use of instruments required in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaur, R.S., Shanker, D., Sudan, V. et al. Associative Genetic Diversity of RoTat 1.2 VSG in Different Trypanosoma evansi Isolates. Acta Parasit. 66, 199–204 (2021). https://doi.org/10.1007/s11686-020-00273-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00273-4