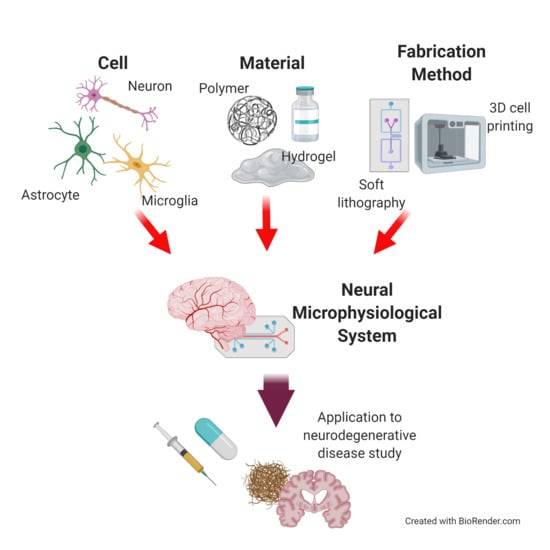
Microphysiological Systems for Neurodegenerative Diseases in Central Nervous System
Abstract
:1. Introduction
2. Neural Cells for Neural Microphysiological System (Neural MPS) Design
2.1. Neurons
2.1.1. Human-Induced Pluripotent Stem Cell-Derived Neuron
2.1.2. Human Fetal Tissue-Derived Neuron
2.2. Glial Cells
2.2.1. Astrocyte
2.2.2. Microglia
3. Materials for Neural MPS Design
3.1. Synthetic Biomaterials
3.1.1. Scaffold Materials
- -
- Polyethylene glycol (PEG)-based hydrogel
3.1.2. Conducting Materials
- -
- Polypyrrole
- -
- Graphene
3.2. Natural Source Derived Biomaterial
3.2.1. Hyaluronic Acid (HA)
3.2.2. Matrigel
3.2.3. Collagen
3.2.4. Decellularized Extracellular Matrix (dECM)
4. Manufacturing Method for Neural MPS Design
4.1. Soft Lithography
4.2. 3D Bioprinting
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, D.; Jackson, R.; Paul, G.; Shi, J.; Sabbagh, M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin. Investig. Drugs 2017, 26, 735–739. [Google Scholar]
- Zahra, W.; Rai, S.N.; Birla, H.; Singh, S.S.; Dilnashin, H.; Rathore, A.S.; Singh, S.P. The Global Economic Impact of Neurodegenerative Diseases: Opportunities and Challenges. In Bioeconomy for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 333–345. [Google Scholar]
- Oxford, A.E.; Stewart, E.S.; Rohn, T.T. Clinical Trials in Alzheimer’s Disease: A Hurdle in the Path of Remedy. Int. J. Alzheimer’s Dis 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Katsuno, M.; Sahashi, K.; Iguchi, Y.; Hashizume, A. Preclinical progression of neurodegenerative diseases. Nagoya J. Med. Sci. 2018, 80, 289. [Google Scholar] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [PubMed]
- Aguzzi, A.; O’connor, T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar]
- Scearce-Levie, K.; Sanchez, P.E.; Lewcock, J.W. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat. Rev. Drug Discov. 2020, 447–462. [Google Scholar] [CrossRef]
- Young, J.E.; Goldstein, L.S. Alzheimer’s disease in a dish: Promises and challenges of human stem cell models. Hum. Mol. Genet. 2012, 21, R82–R89. [Google Scholar]
- Choi, S.H.; Kim, Y.H.; Quinti, L.; Tanzi, R.E.; Kim, D.Y. 3D culture models of Alzheimer’s disease: A road map to a “cure-in-a-dish”. Mol. Neurodegener. 2016, 11, 75. [Google Scholar]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. Altex 2016, 33, 272. [Google Scholar]
- Liu, L.; Koo, Y.; Akwitti, C.; Russell, T.; Gay, E.; Laskowitz, D.T.; Yun, Y. Three-dimensional (3D) brain microphysiological system for organophosphates and neurochemical agent toxicity screening. PLoS ONE 2019, 14, e0224657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offeddu, G.S.; Shin, Y.; Kamm, R.D. Microphysiological models of neurological disorders for drug development. Curr. Opin. Biomed. Eng. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Haring, A.P.; Sontheimer, H.; Johnson, B.N. Microphysiological human brain and neural systems-on-a-chip: Potential alternatives to small animal models and emerging platforms for drug discovery and personalized medicine. Stem Cell Rev. Rep. 2017, 13, 381–406. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamies, D.; Barrera, P.; Block, K.; Makri, G.; Kumar, A.; Wiersma, D.; Smirnova, L.; Zhang, C.; Bressler, J.; Christian, K.M. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. Altex 2017, 34, 362. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Wang, C. Organoids and Microphysiological Systems: New Tools for Ophthalmic Drug Discovery. Front Pharmacol. 2020, 11, 407. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, R.S. Human Brain Function; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. USA 2004, 101, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Simons, J.S.; Spiers, H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003, 4, 637–648. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [Green Version]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [PubMed]
- Li, L.; Chao, J.; Shi, Y. Modeling neurological diseases using iPSC-derived neural cells. Cell Tissue Res. 2018, 371, 143–151. [Google Scholar] [CrossRef]
- Du, Z.-W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.-L.; Zhong, X.; Fan, F.; Zhang, S.-C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.-Y.; Zhang, S.-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009, 4, 1295. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.A.; Fairbanks, L.A.; Tekin, S.; Vinters, H.V.; Cummings, J.L. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J. Geriatr. Psychiatry Neurol. 2007, 20, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.; Dumanchin, C.; Hannequin, D.; Dubois, B.; Belliard, S.; Puel, M.; Thomas-Anterion, C.; Michon, A.; Martin, C.; Charbonnier, F. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999, 65, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brickell, K.L.; Steinbart, E.J.; Rumbaugh, M.; Payami, H.; Schellenberg, G.D.; Van Deerlin, V.; Yuan, W.; Bird, T.D. Early-onset Alzheimer disease in families with late-onset Alzheimer disease: A potential important subtype of familial Alzheimer disease. Arch. Neurol. 2006, 63, 1307–1311. [Google Scholar] [CrossRef]
- Nieweg, K.; Andreyeva, A.; Van Stegen, B.; Tanriöver, G.; Gottmann, K. Alzheimer’s disease-related amyloid-β induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 2015, 6, e1709. [Google Scholar] [PubMed]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2019, 148–167. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, G.X.; Rose, S.E.; Knupp, A.; Nicholson, D.A.; Keene, C.D.; Young, J.E. The application of in vitro-derived human neurons in neurodegenerative disease modeling. J. Neurosci. Res. 2020. [Google Scholar] [CrossRef]
- Ortuño-Costela, M.d.C.; Cerrada, V.; García-López, M.; Gallardo, M.E. The challenge of bringing iPSCs to the patient. Int. J. Mol. Sci. 2019, 20, 6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Koo, Y.; Russell, T.; Gay, E.; Li, Y.; Yun, Y. Three-dimensional brain-on-chip model using human iPSC-derived GABAergic neurons and astrocytes: Butyrylcholinesterase post-treatment for acute malathion exposure. PLoS ONE 2020, 15, e0230335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, S.; Workman, M.J.; Herland, A.; Svendsen, C.N.; Vatine, G.D. Generation of a Human iPSC-Based Blood-Brain Barrier Chip. JoVE (J. Vis. Exp.) 2020, e60925. [Google Scholar] [CrossRef]
- Dauth, S.; Maoz, B.M.; Sheehy, S.P.; Hemphill, M.A.; Murty, T.; Macedonia, M.K.; Greer, A.M.; Budnik, B.; Parker, K.K. Neurons derived from different brain regions are inherently different in vitro: A novel multiregional brain-on-a-chip. J. Neurophysiol. 2017, 117, 1320–1341. [Google Scholar] [CrossRef]
- Tukker, A.M.; Wijnolts, F.M.; de Groot, A.; Westerink, R.H. Human iPSC-derived neuronal models for in vitro neurotoxicity assessment. Neurotoxicology 2018, 67, 215–225. [Google Scholar] [CrossRef]
- Dolmetsch, R.; Geschwind, D.H. The human brain in a dish: The promise of iPSC-derived neurons. Cell 2011, 145, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, C.; Lesimple, P.; Bukowiecki, R.; Zink, A.; Inak, G.; Mlody, B.; Singh, M.; Semtner, M.; Mah, N.; Auré, K. Human iPSC-derived neural progenitors are an effective drug discovery model for neurological mtDNA disorders. Cell Stem Cell 2017, 20, 659–674.e9. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Liddelow, S.A. Astrocytes and microglia: Models and tools. J. Exp. Med. 2019, 216, 71–83. [Google Scholar] [CrossRef]
- Miller, D.W.; Cookson, M.R.; Dickson, D.W. Glial cell inclusions and the pathogenesis of neurodegenerative diseases. Neuron Glia Biol. 2004, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Yao, L. Glial Cell Engineering in Neural Regeneration; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Galland, F.; Seady, M.; Taday, J.; Smaili, S.S.; Gonçalves, C.A.; Leite, M.C. Astrocyte culture models: Molecular and function characterization of primary culture, immortalized astrocytes and C6 glioma cells. Neurochem. Int. 2019, 131, 104538. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [PubMed] [Green Version]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [PubMed]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 483–491. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Lauranzano, E.; Campo, E.; Rasile, M.; Molteni, R.; Pizzocri, M.; Passoni, L.; Bello, L.; Pozzi, D.; Pardi, R.; Matteoli, M. A Microfluidic Human Model of Blood–Brain Barrier Employing Primary Human Astrocytes. Adv. Biosyst. 2019, 3, 1800335. [Google Scholar] [CrossRef]
- Ikeshima-Kataoka, H. Neuroimmunological implications of AQP4 in astrocytes. Int. J. Mol. Sci. 2016, 17, 1306. [Google Scholar] [CrossRef]
- Jullienne, A.; Fukuda, A.M.; Ichkova, A.; Nishiyama, N.; Aussudre, J.; Obenaus, A.; Badaut, J. Modulating the water channel AQP4 alters miRNA expression, astrocyte connectivity and water diffusion in the rodent brain. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Placone, A.L.; McGuiggan, P.M.; Bergles, D.E.; Guerrero-Cazares, H.; Quiñones-Hinojosa, A.; Searson, P.C. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials 2015, 42, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simão, D.; Terrasso, A.P.; Teixeira, A.P.; Brito, C.; Sonnewald, U.; Alves, P.M. Functional metabolic interactions of human neuron-astrocyte 3D in vitro networks. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placone, A.L.; Quiñones-Hinojosa, A.; Searson, P.C. The role of astrocytes in the progression of brain cancer: Complicating the picture of the tumor microenvironment. Tumor Biol. 2016, 37, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Packard, J.A.; Leach, J.B.; Powell, E.M. Three-dimensional environment sustains morphological heterogeneity and promotes phenotypic progression during astrocyte development. Tissue Eng. Part A 2016, 22, 885–898. [Google Scholar] [CrossRef] [Green Version]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193. [Google Scholar]
- Park, J.; Nicoll, J.A.; Holmes, C. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar]
- Timmerman, R.; Burm, S.M.; Bajramovic, J.J. An overview of in vitro methods to study microglia. Front. Cell. Neurosci. 2018, 12, 242. [Google Scholar]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar]
- Letourneau, P.C.; Condic, M.L.; Snow, D.M. Interactions of developing neurons with the extracellular matrix. J. Neurosci. 1994, 14, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venkateswaran, S.; Higuera, G.A.; Nath, S.; Shpak, G.; Matray, J.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Kushner, S.A.; Bradley, M. Synthetic Polymers Provide a Robust Substrate for Functional Neuron Culture. Adv. Healthc. Mater. 2020, 9, 1901347. [Google Scholar]
- Tourniaire, G.; Collins, J.; Campbell, S.; Mizomoto, H.; Ogawa, S.; Thaburet, J.-F.; Bradley, M. Polymer microarrays for cellular adhesion. Chem. Commun. 2006, 2118–2120. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar]
- Burdick, J.A.; Ward, M.; Liang, E.; Young, M.J.; Langer, R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 2006, 27, 452–459. [Google Scholar] [CrossRef]
- Piantino, J.; Burdick, J.; Goldberg, D.; Langer, R.; Benowitz, L. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp. Neurol. 2006, 201, 359–367. [Google Scholar] [CrossRef]
- Lampe, K.J.; Bjugstad, K.B.; Mahoney, M.J. Impact of degradable macromer content in a poly (ethylene glycol) hydrogel on neural cell metabolic activity, redox state, proliferation, and differentiation. Tissue Eng. Part A 2010, 16, 1857–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, S.; Salerno, S.; Piscioneri, A.; Tasselli, F.; Drioli, E.; De Bartolo, L. Neuronal membrane bioreactor as a tool for testing crocin neuroprotective effect in Alzheimer’s disease. Chem. Eng. J. 2016, 305, 69–78. [Google Scholar] [CrossRef]
- Moreno, E.L.; Hachi, S.; Hemmer, K.; Trietsch, S.J.; Baumuratov, A.S.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Fleming, R.M.J.L.o.a.C. Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture. Lab Chip 2015, 15, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Hsu, C.-C.; Nguyen, L.T.B.; Ye, H.; Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bonmassar, G.; Lee, S.W.; Freeman, D.K.; Polasek, M.; Fried, S.I.; Gale, J.T. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 68, 411–422. [Google Scholar]
- Zhang, Q.; Esrafilzadeh, D.; Crook, J.M.; Kapsa, R.; Stewart, E.M.; Tomaskovic-Crook, E.; Wallace, G.G.; Huang, X.-F. Electrical stimulation using conductive polymer polypyrrole counters reduced neurite outgrowth of primary prefrontal cortical neurons from NRG1-KO and DISC1-LI Mice. Sci. Rep. 2017, 7, 42525. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef]
- Palermo, V. Not a molecule, not a polymer, not a substrate… the many faces of graphene as a chemical platform. Chem. Commun. 2013, 49, 2848–2857. [Google Scholar] [CrossRef]
- Solanki, A.; Chueng, S.T.D.; Yin, P.T.; Kappera, R.; Chhowalla, M.; Lee, K.B. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv. Mater. 2013, 25, 5477–5482. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wu, L.; Cheng, J.; Huang, S.; Cai, Q.; Jin, Q.; Zhao, J. Graphene microelectrode arrays for neural activity detection. J. Boil. Phys. 2015, 41, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Bramini, M.; Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F.J. Interfacing graphene-based materials with neural cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef]
- El Merhie, A.; Ito, D.; Colombi, I.; Keshavan, S.; Mishra, N.; Miseikis, V.; Diaspro, A.; Coletti, C.; Chiappalone, M.; Dante, S.; et al. Single layer graphene functionalized MEA for enhanced detection of neuronal network development. Sens. Actuators B Chem. 2018, 277, 224–233. [Google Scholar]
- Sakai, K.; Teshima, T.F.; Nakashima, H.; Ueno, Y. Graphene-based neuron encapsulation with controlled axonal outgrowth. Nanoscale 2019, 11, 13249–13259. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar]
- Chen, R.; Canales, A.; Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23. [Google Scholar] [CrossRef]
- Barros, C.S.; Franco, S.J.; Müller, U. Extracellular matrix: Functions in the nervous system. Cold Spring Harb. Perspect. Biol. 2011, 3, a005108. [Google Scholar] [CrossRef] [Green Version]
- Schizas, N.; Rojas, R.; Kootala, S.; Andersson, B.; Pettersson, J.; Hilborn, J.; Hailer, N.P. Hyaluronic acid-based hydrogel enhances neuronal survival in spinal cord slice cultures from postnatal mice. J. Biomater. Appl. 2014, 28, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Hsueh, C.-H. Neuronal production from induced pluripotent stem cells in self-assembled collagen-hyaluronic acid-alginate microgel scaffolds with grafted GRGDSP/Ln5-P4. Mater. Sci. Eng. C 2017, 76, 760–774. [Google Scholar] [CrossRef]
- Tay, A.; Rojas, R.; Kootala, S.; Andersson, B.; Pettersson, J.; Hilborn, J.; Hailer, N.P. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: A state-of-the-art review. Comput. Struct. Biotechnol. J. 2018, 16, 488–502. [Google Scholar] [CrossRef]
- Rauti, R.; Renous, N.; Maoz, B.M. Mimicking the Brain Extracellular Matrix in Vitro: A Review of Current Methodologies and Challenges. Isr. J. Chem. 2019. [Google Scholar] [CrossRef]
- Ruoslahti, E. Brain extracellular matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus 2012, 2, 278–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Xu, R.; Duan, B.; Jiang, P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B 2017, 5, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Yan, W.; Liu, W.; Qi, J.; Fang, Q.; Fan, Z.; Sun, G.; Han, Y.; Zhang, D.; Xu, L.; Wang, M. A three-dimensional culture system with Matrigel promotes purified spiral ganglion neuron survival and function in vitro. Mol. Neurobiol. 2018, 55, 2070–2084. [Google Scholar] [CrossRef]
- Jang, J.M.; Tran, S.-H.-T.; Na, S.C.; Jeon, N.L. Engineering controllable architecture in matrigel for 3D cell alignment. ACS Appl. Mater. Interfaces 2015, 7, 2183–2188. [Google Scholar] [CrossRef]
- Janzen, D.; Bakirci, E.; Wieland, A.; Martin, C.; Dalton, P.D.; Villmann, C. Cortical Neurons form a Functional Neuronal Network in a 3D Printed Reinforced Matrix. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef]
- Bang, S.; Na, S.; Jang, J.M.; Kim, J.; Jeon, N.L. Engineering-aligned 3D neural circuit in microfluidic device. Adv. Healthc. Mater. 2016, 5, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, S.H.; D’avanzo, C.; Hebisch, M.; Sliwinski, C.; Bylykbashi, E.; Washicosky, K.J.; Klee, J.B.; Brüstle, O.; Tanzi, R.E. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat. Protoc. 2015, 10, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Im, S.-K.; Oh, S.-J.; Jeong, S.; Yoon, E.-S.; Lee, C.J.; Choi, N.; Hur, E.-M. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat. Commun. 2017, 8, 1–16. [Google Scholar]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lee, J.S.; Kim, J.; Min, S.; Wi, S.; Yu, J.H.; Chang, G.-E.; Cho, A.-N.; Choi, Y.; Ahn, D.-H. Three-dimensional brain-like microenvironments facilitate the direct reprogramming of fibroblasts into therapeutic neurons. Nat. Biomed. Eng. 2018, 2, 522–539. [Google Scholar]
- Sin, A.; Chin, K.C.; Jamil, M.F.; Kostov, Y.; Rao, G.; Shuler, M.L. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol. Prog. 2004, 20, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Kang, M.; Bang, S.; Park, D.; Kim, J.; Sim, S.J.; Chang, S.; Jeon, N.L. Microfluidic neural axon diode. Technology 2016, 4, 240–248. [Google Scholar] [CrossRef]
- Honegger, T.; Thielen, M.I.; Feizi, S.; Sanjana, N.E.; Voldman, J. Microfluidic neurite guidance to study structure-function relationships in topologically-complex population-based neural networks. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood–brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41. [Google Scholar]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, R.; Leenders, K.L.; Van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Van Der Helm, M.W.; Van Der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [Green Version]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow. Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef]
- Phan, D.T.; Bender, R.H.F.; Andrejecsk, J.W.; Sobrino, A.; Hachey, S.J.; George, S.C.; Hughes, C.C. Blood–brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood–central nervous system interface. Exp. Boil. Med. 2017, 242, 1669–1678. [Google Scholar] [CrossRef]
- Cho, C.-F.; Cho, C.-F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D neural tissue models: From spheroids to bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Willerth, S.M. 3-D bioprinting of neural tissue for applications in cell therapy and drug screening. Front. Bioeng. Biotechnol. 2017, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef]
- Campos, P.B.; Paulsen, B.S.; Rehen, S.K. Accelerating neuronal aging in in vitro model brain disorders: A focus on reactive oxygen species. Front. Aging Neurosci. 2014, 6, 292. [Google Scholar]
- Grenier, K.; Kao, J.; Diamandis, P. Three-dimensional modeling of human neurodegeneration: Brain organoids coming of age. Mol. Psychiatry 2020, 25, 254–274. [Google Scholar]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Park. Dis. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.M.; Carelli, S.; Cereda, C. From neuronal differentiation of iPSCs to 3D neuro-organoids: Modelling and therapy of neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-T.; Bendriem, R.M.; Wu, W.W.; Shen, R.-F. 3D brain Organoids derived from pluripotent stem cells: Promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Bruno, K.L.D.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.-L. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul. Toxicol. Pharmacol. 2020, 104662. [Google Scholar] [CrossRef] [PubMed]
- Ewart, L.; Dehne, E.-M.; Fabre, K.; Gibbs, S.; Hickman, J.; Hornberg, E.; Ingelman-Sundberg, M.; Jang, K.-J.; Jones, D.R.; Lauschke, V.M. Application of microphysiological systems to enhance safety assessment in drug discovery. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 65–82. [Google Scholar] [CrossRef] [PubMed]






| Materials | Source | Advantages | Limitation |
|---|---|---|---|
| Hyaluronic acid (HA) | Rooster combs, bovine eyes, streptococcus qui [91,92,93]. | High accessibility to isolate the material Low batch-to-batch variation Stable structure | Low cell attachment on the HA [94] |
| Matrigel | Engel-Holm-Swarm mouse sarcoma cells [94] | Various ECM proteins which are abundant in the CNS | Batch-to-batch variation Low mechanical property (degradability) |
| Collagen | Pig, rat, fish | High controllability for mechanical properties [95]. | Lack of other critical proteins of CNS Different collagen type with main collagen in CNS |
| Decelluarized extracellular matrix(dECM) | Pig, human, mouse, etc. | Favorable to reproduce physiolological environment of the CNS (protein composition, physical properties) Enhancing neurogenic effects for neural cells [96,97]. | Batch-to-batch variation Unrevealed protein composition |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Yi, H.-G.; Jang, J.; Cho, D.-W. Microphysiological Systems for Neurodegenerative Diseases in Central Nervous System. Micromachines 2020, 11, 855. https://doi.org/10.3390/mi11090855
Bae M, Yi H-G, Jang J, Cho D-W. Microphysiological Systems for Neurodegenerative Diseases in Central Nervous System. Micromachines. 2020; 11(9):855. https://doi.org/10.3390/mi11090855
Chicago/Turabian StyleBae, Mihyeon, Hee-Gyeong Yi, Jinah Jang, and Dong-Woo Cho. 2020. "Microphysiological Systems for Neurodegenerative Diseases in Central Nervous System" Micromachines 11, no. 9: 855. https://doi.org/10.3390/mi11090855