- 1Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, China
- 2Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Shanghai, China
Purpose: Lefamulin is a novel antibiotic approved by the U.S. Food and Drug Administration in 2019 for the treatment of community-acquired bacterial pneumonia (CABP). In this study we evaluated the in vitro antimicrobial activity of lefamulin in order to better understand its antibiogram.
Methods: The test strains were isolated from patients across China during the period from 2017 to 2019, including 634 strains of respiratory pathogens. The minimum inhibitory concentrations (MICs) of lefamulin and comparators were determined by broth microdilution method.
Results: Lefamulin showed potent activity against Streptococcus pneumoniae and Staphylococcus evidenced by 100% inhibition at 0.25 mg/L, and favorable MIC50/90 (0.125/0.125 mg/L) against S. pneumoniae (penicillin MIC ≥ 2 mg/L), MIC50/90 (≤0.015/0.125 mg/L) against methicillin-resistant S. aureus, and MIC50/90 (≤0.015/0.06 mg/L) against methicillin-resistant S. epidermidis. Lefamulin also had good activity against Streptococcus pyogenes and Streptococcus agalactia (MIC50/90: ≤0.015/≤0.015 mg/L), β-lactamase-producing Haemophilus influenzae (MIC50/90: 0.5/1 mg/L), β-lactamase-negative H. influenzae (MIC50/90: 1/1 mg/L), Moraxella catarrhalis (MIC50/90: 0.25/0.25 mg/L), and Mycoplasma pneumoniae (MIC50/90: 0.03/0.03 mg/L) regardless of resistance to azithromycin. Lefamulin was generally more active than the comparators against the test strains.
Conclusion: In summary, lefamulin has good and broad-spectrum coverage of respiratory pathogens (methicillin-sensitive and -resistant Staphylococcus, S. pneumoniae, β-hemolytic Streptococcus, H. influenzae, M. catarrhalis and M. pneumoniae). In vitro activity supports the use of lefamulin in the treatment of CABP in China.
Introduction
Pleuromutilin is a natural antimicrobial substance first found in 1950s. It can be obtained from Clitopilus scyphoides, Clitopilus passeckerianus, or other Clitopilus species in basidiomycota. Lefamulin is the first-in-class semi-synthetic pleuromutilin antibiotic for systemic use. Its molecular formula is C28H45NO5S (molecular weight 567.79 g). Lefamulin inhibits bacterial protein synthesis by binding to “A” and “P” sites of the peptidyl transferase center (PTC) of the 23s rRNA of the 50S ribosomal subunit of bacterial cell. The binding is through the mutilin core and C-14 side chain in the forms of hydrogen bonds, hydrophobic interactions, and conformational change to prevent correct orientation of tRNA’s 3’-CCA ends for peptide transfer (Veve and Wagner, 2018; Rodvold, 2019). The resistance to lefamulin may be related to the mutations in rplC gene and cfr gene of Staphylococcus aureus, Vga (AV) coded by the transposon Tn5406 and vga(A) carried by plasmids (encoding ABC transporter) (Mendes et al., 2019; Rodvold, 2019). So far, it is known that lefamulin has no cross resistance to the antimicrobial agents in clinical use.
Studies have shown that lefamulin has good coverage of the pathogens of community-acquired respiratory tract infections, including antibiotic-resistant strains, such as penicillin-resistant Streptococcus pneumoniae (PRSP), macrolide-resistant Mycoplasma pneumoniae, and methicillin-resistant S. aureus (MRSA) (Veve and Wagner, 2018; Rodvold, 2019). In August 2019, lefamulin was approved by the U.S. Food and Drug Administration (FDA) for the treatment of community-acquired bacterial pneumonia (CABP) patients based on its good pharmacodynamic results, pharmacokinetic, and safety profiles in clinical trials.
The antibacterial spectrum and activity of lefamulin have been studied in the United States and Europe (Paukner et al., 2013, 2019), but it is not clear about its antimicrobial activity against the clinical isolates in China. For better understanding the antimicrobial activity of lefamulin against the common respiratory pathogens recently isolated in China, we studied the in vitro activity of lefamulin against a broad range of respiratory pathogens.
Materials and Methods
A total of 634 non-duplicate strains of respiratory pathogens were tested, including 580 strains of bacteria and 54 strains of Mycoplasma pneumoniae. These strains were isolated from 29 hospitals across China, representing 23 provinces and municipalities, during the period from October 2017 to July 2019. Specifically, the test strains included S. aureus (n = 121), S. epidermidis (n = 30), β-lactamase-producing Haemophilus influenzae (n = 48), β-lactamase-negative H. influenzae (n = 48), Haemophilus parainfluenzae (n = 10), Moraxella catarrhalis (n = 54), S. pneumoniae (n = 172), Streptococcus pyogenes (n = 30), and Streptococcus agalactiae (n = 13). All the strains were re-identified before susceptibility testing. Species identification was confirmed by MALDI-TOF/MS system (bioMérieux, France), and antimicrobial susceptibility testing were controlled with reference strains S. aureus ATCC29213, S. pneumoniae ATCC49619, H. influenzae ATCC49247, and M. pneumoniae ATCC 29342.
The minimum inhibitory concentrations (MICs) of lefamulin and the comparators were determined by broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) (2018) M07-11th Edition (CLSI, 2018). The MICs against M. pneumoniae were measured according to the methods for antimicrobial susceptibility testing for human mycoplasmas described in CLSI document M43-A (2011) (CLSI, 2011). The antimicrobial comparators included tigecycline, moxifloxacin, linezolid, penicillin, ampicillin, oxacillin, ceftriaxone, levofloxacin, vancomycin, trimethoprim-sulfamethoxazole, erythromycin, and azithromycin. The concentrations of the test antimicrobial agents ranged from 32 mg/L to 0.015 mg/L.
WHONET 5.6 software and the breakpoints of CLSI M100-29th Edition (CLSI, 2019) were used to interpret and analyze the results of antimicrobial susceptibility test. Lefamulin and tigecycline were analyzed according to the breakpoints recommended by FDA1. The breakpoints of lefamulin was ≤0.25 mg/L active against methicillin-sensitive S. aureus, ≤0.5 mg/L against S. pneumoniae, and ≤2 mg/L against H. influenzae. The breakpoint of tigecycline was ≤0.5 mg/L active against S. aureus, and ≤0.25 mg/L against H. influenzae.
Ethics Statement
The study protocol was approved by the Ethics Committee of Huashan Hospital, Fudan University (Number: 2019-319).
Results
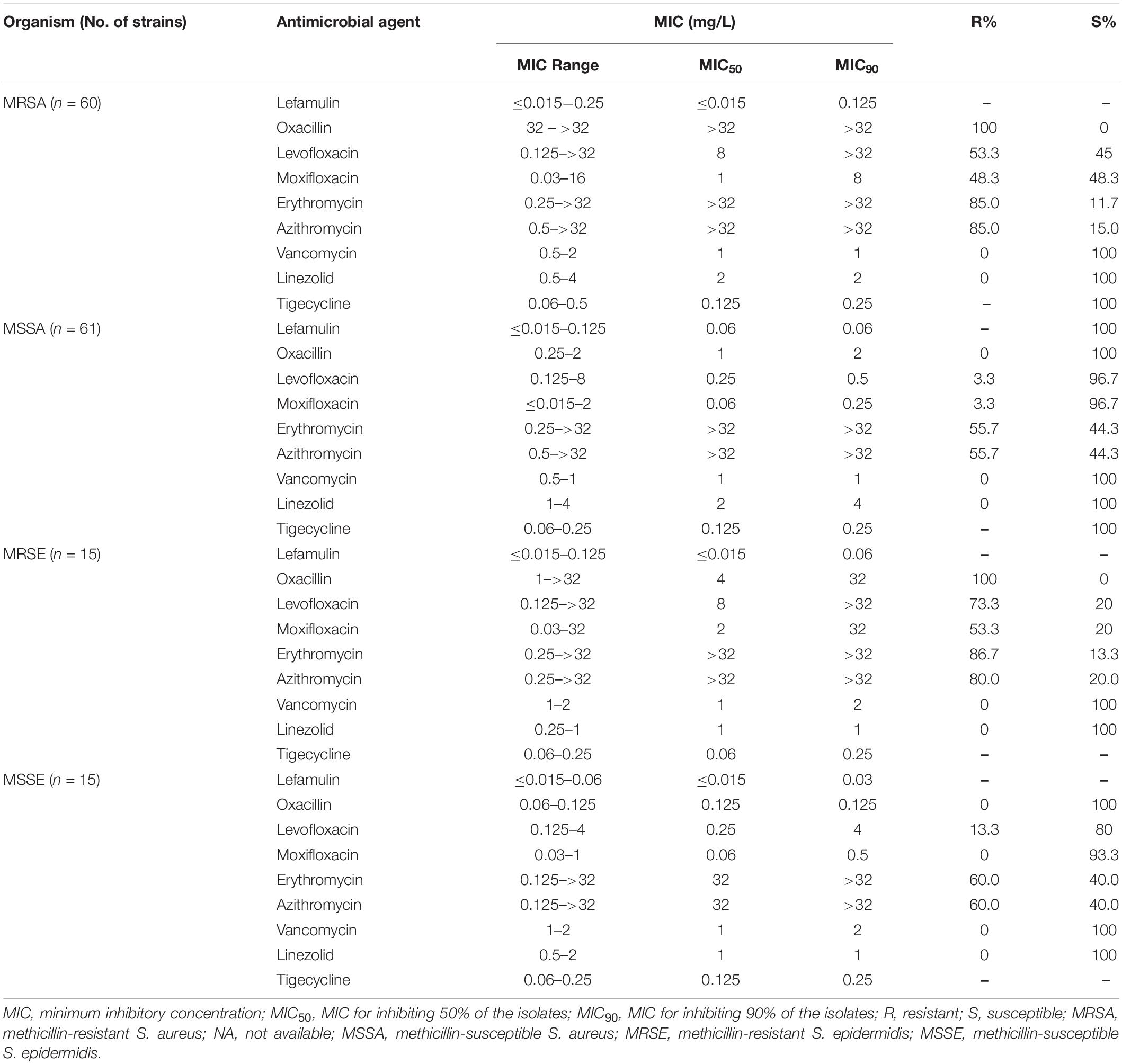
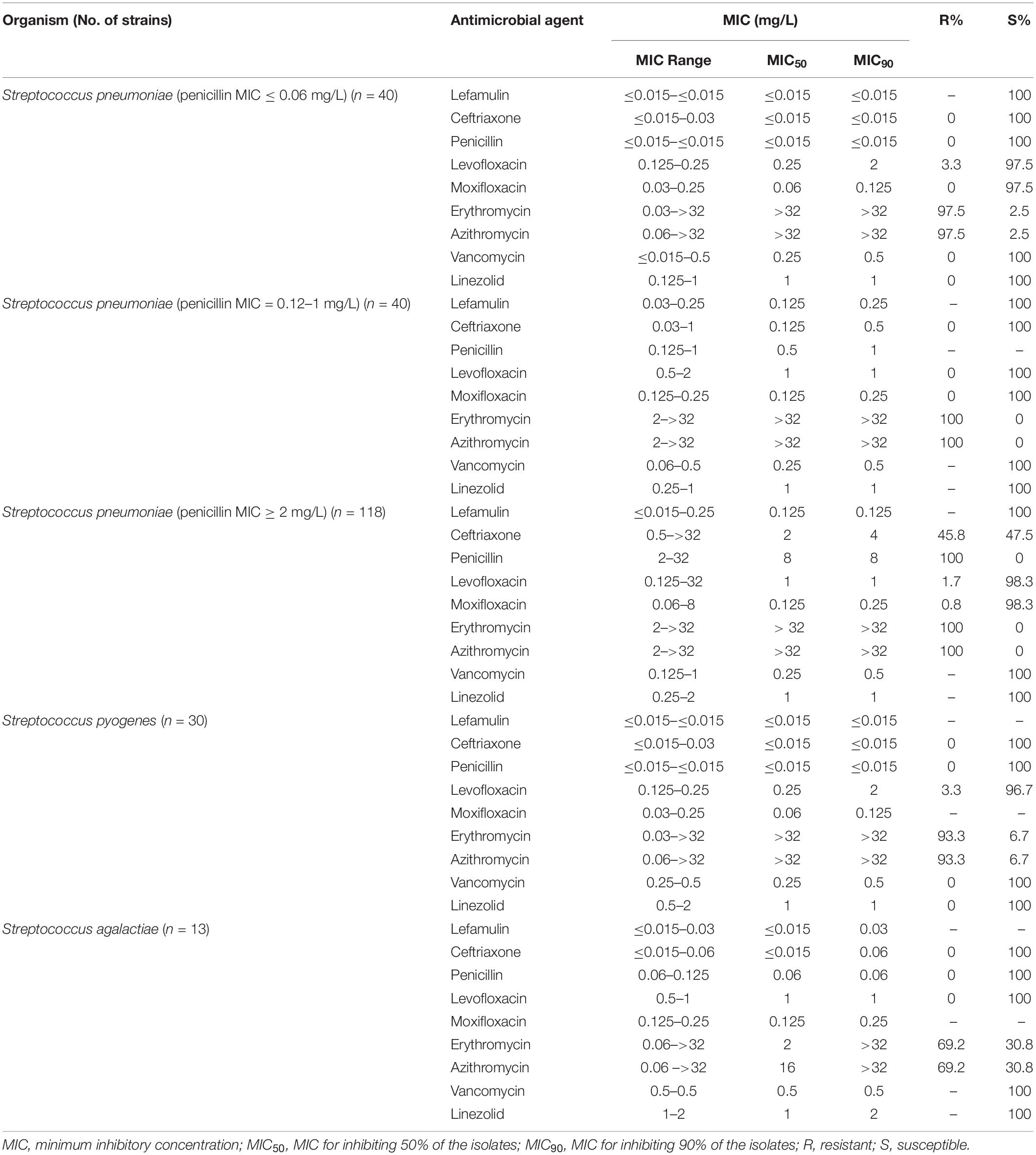
Lefamulin at 0.25 mg/L inhibited the growth of all Staphylococcus strains (Table 1 and Figure 1). The MIC90 value of lefamulin was 0.125 mg/L against MRSA, 0.06 mg/L against methicillin-resistant S. epidermidis (MRSE), 0.06 mg/L against methicillin-sensitive S. aureus (MSSA), and 0.03 mg/L against methicillin-sensitive S. epidermidis (MSSE). Lefamulin displayed MIC values ranging from ≤0.015 mg/L to 0.25 mg/L (MIC90: ≤0.25 mg/L) against 172 strains of S. pneumoniae, including penicillin-susceptible (PSSP) strains (penicillin MIC ≤0.06 mg/L), penicillin-intermediate (PISP) strains (penicillin MIC: 0.125 mg/L–1 mg/L), and penicillin-resistant (PRSP) strains (penicillin MIC ≥ 2 mg/L). Lefamulin inhibited the growth of all PSSP strains at ≤0.015 mg/L and all PISP and PRSP strains at 0.25 mg/L. The MIC50/90 values of lefamulin were ≤0.015/≤0.015 mg/L against S. pyogenes and ≤0.015/0.06 mg/L against S. agalactiae. Lefamulin inhibited the growth of all the S. pyogenes and S. agalactiae strains at 0.06 mg/L (Table 2 and Figure 1).
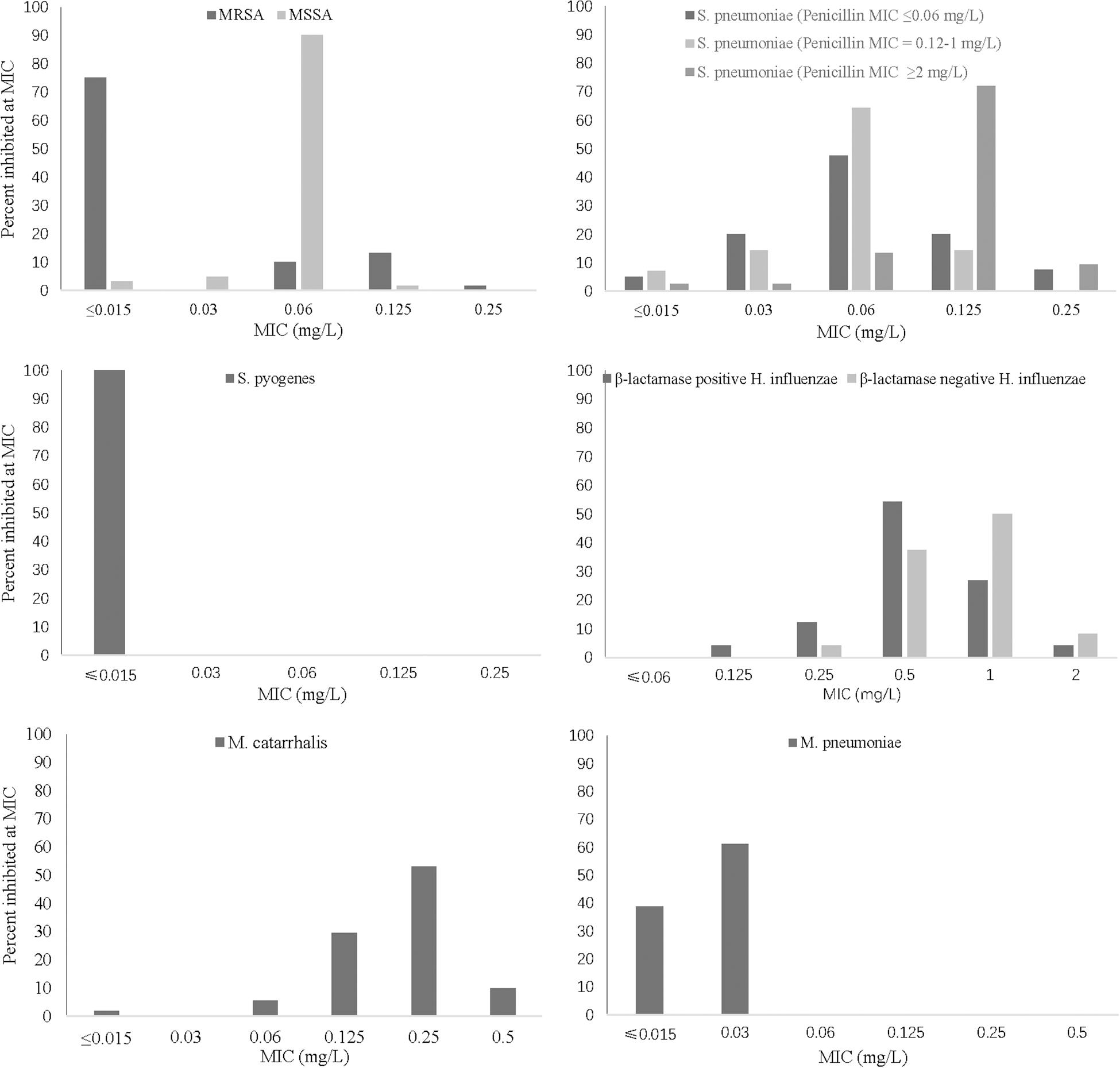
Figure 1. MIC frequency distribution of lefamulin against respiratory pathogens. MRSA (n = 60), MSSA (n = 61), S. pneumoniae strains (penicillin MIC ≤ 0.06 mg/L, n = 40; penicillin MIC = 0.12–1 mg/L, n = 40; penicillin MIC ≥ 2 mg/L, n = 118), S. pyogenes (n = 30), H. influenzae (β-lactamase-positive strains, n = 48; β-lactamase-negative strains, n = 48), M. catarrhalis (n = 54), and M. pneumoniae (n = 54). MIC, minimum inhibitory concentration; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
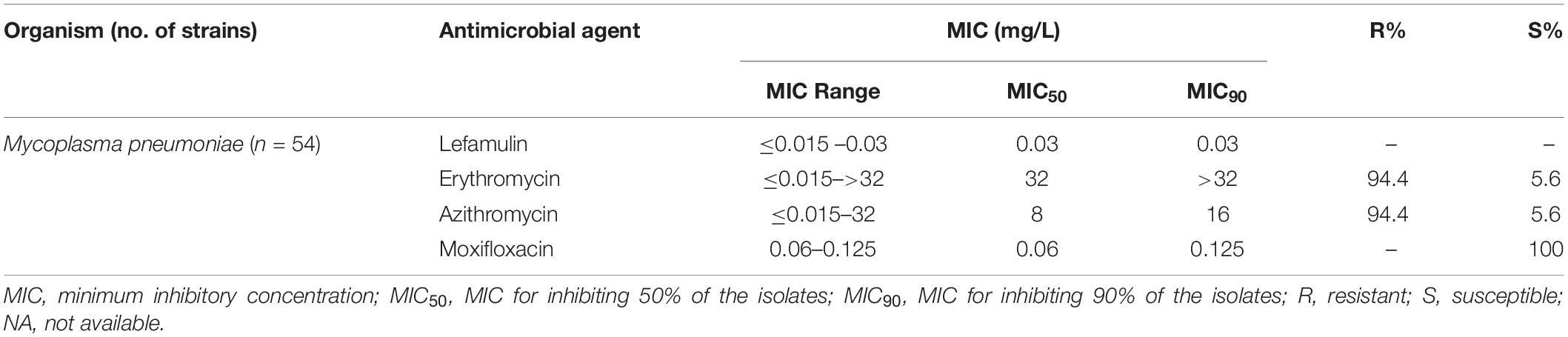
The MIC50/90 values of lefamulin ≤ 1/ ≤ 1 mg/L against H. influenzae and H. parainfluenzae, regardless of β-lactamase production. Lefamulin inhibited the growth of all the Haemophilus strains at 2 mg/L (Table 3 and Figure 1). Lefamulin showed MIC50/90 of 0.25/0.25mg/L against M. catarrhalis. Lefamulin inhibited the growth of all M. catarrhalis strains at 0.5 mg/L (Table 3 and Figure 1).
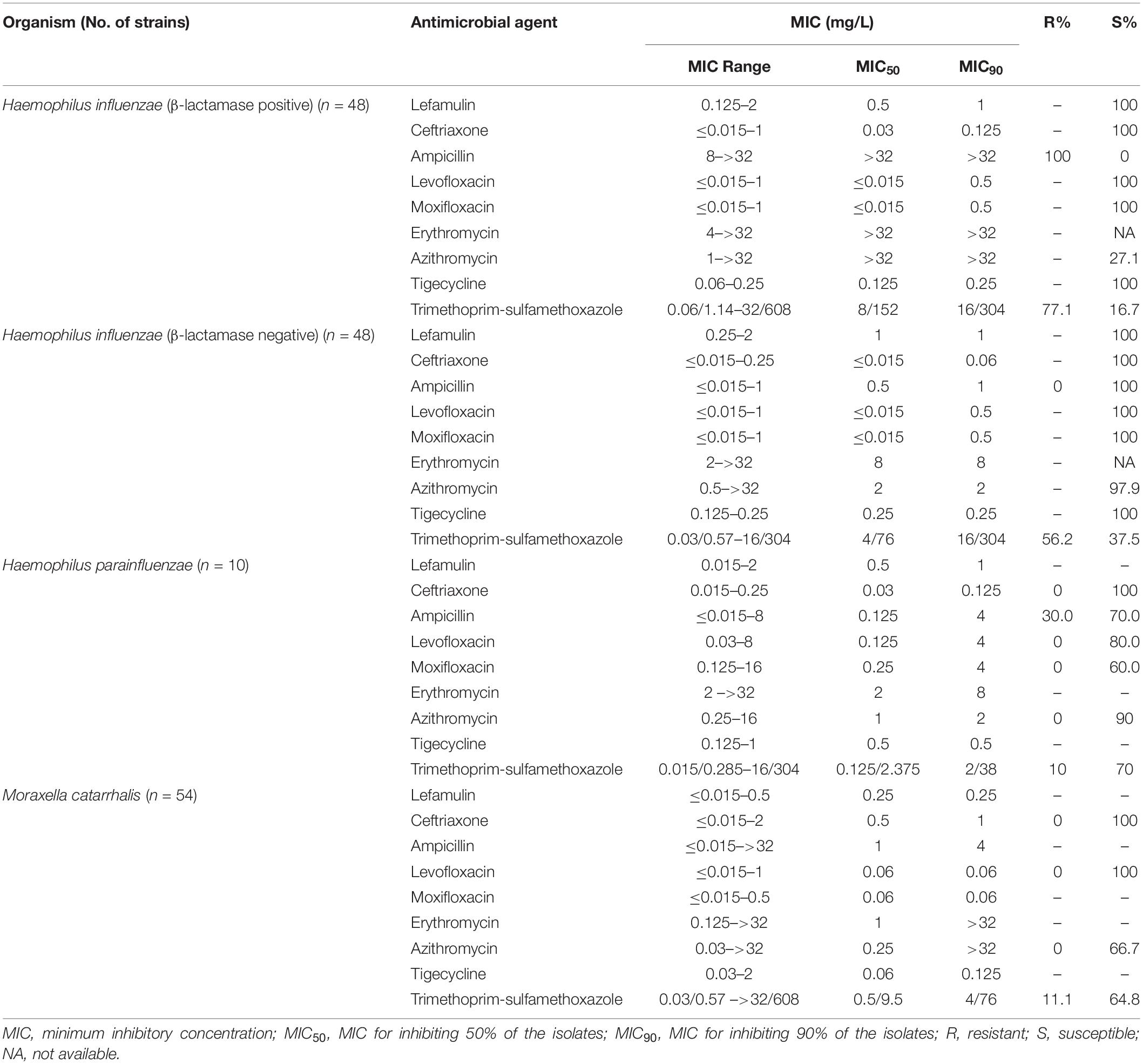
Table 3. In vitro activity of lefamulin and comparators against Haemophilus influenzae and Moraxella catarrhalis.
Lefamulin inhibited the growth of all M. pneumoniae strains at 0.03 mg/L. The MIC ranged from ≤0.015 to 0.03 mg/L (MIC50/90: 0.03/0.03 mg/L). Its activity was comparable to moxifloxacin and significantly superior to erythromycin and azithromycin (Table 4 and Figure 1).
Discussion
In the present study, lefamulin displayed excellent antimicrobial activity against all the respiratory pathogens, including MRSA, MSSA, MRSE, MSSE, S. pneumoniae, β-hemolytic Streptococcus, Haemophilus, M. catarrhalis, and M. pneumoniae. Our results are consistent with the reports of Susanne Paukner et al. on the antimicrobial activity of lefamulin against 1,473 and 2,661 strains of S. pneumoniae, 3,923 and 2,919 strains of S. aureus in the SENTRY Antimicrobial Surveillance Program 2010 and 2015–2016 (Paukner et al., 2013, 2019). The MIC90 of lefamulin was 0.25 mg/L and 0.12 mg/L against S. pneumoniae, regardless of resistance to penicillin, ceftriaxone and/or levofloxacin. The MIC50/90 was 0.12/0.12 mg/L against MRSA and MSSA. They also reported that the MIC value of lefamulin was 2–>16 mg/L against two MRSA isolates and 5 MSSA isolates in 2010, whereas the MIC value of lefamulin against 11 S. aureus isolates in 2015–2016 was higher than its epidemiological cutoff value. However, all the Staphylococcus strains tested in the present study were sensitive to lefamulin. All the Staphylococcus strains were also susceptible to tigecycline, vancomycin, and linezolid. However, lefamulin inhibited the growth of all Staphylococcus strains at concentration of ≤0.25 mg/L, which is far lower than the concentration of 1–2, 1–4, and 0.25–0.5 mg/L required by the above three comparators for 100% inhibition of bacterial growth. Lefamulin was also superior to quinolones (only inhibited 80–96.7% of the strains) in this respect.
Lefamulin also displayed high antimicrobial activity against Haemophilus and M. catarrhalis. Lefamulin was comparable to ceftriaxone in activity against S. pneumoniae strains (PSSP, PISP) and β-hemolytic Streptococcus, but better than ceftriaxone against PRSP, better than penicillin against PISP and PRSP, and similar to penicillin against β-hemolytic Streptococcus. Lefamulin had similar activity as moxifloxacin, vancomycin, and linezolid against Streptococcus. It inhibited the growth of all Streptococcus species at 0.125 mg/L, which was lower than the above mentioned three agents. Lefamulin was significantly better than erythromycin and azithromycin in the activity against S. pneumoniae and β-hemolytic Streptococcus.
In this study, lefamulin also had good antimicrobial effect on the gram-negative bacilli commonly found in CABP. Lefamulin was similar to ceftriaxone, tigecycline, levofloxacin, and moxifloxacin, and better than ampicillin, azithromycin, and trimethoprim-sulfamethoxazole in the activity against β-lactamase-producing H. influenzae and M. catarrhalis. As for the β-lactamase-negative strains, lefamulin provided significantly better activity than azithromycin. Lefamulin was comparable to tigecycline, ceftriaxone, and levofloxacin, and significantly superior to azithromycin and trimethoprim-sulfamethoxazole in the activity against M. catarrhalis. These results are consistent with those reports from other countries (Paukner et al., 2013, 2019).
It has been reported that the M. pneumoniae strains isolated from China are highly resistant to macrolides. Our results also confirmed the previous reports. About 94.4% of the 54 M. pneumoniae strains were resistant to erythromycin and azithromycin in this study. However, lefamulin still showed MIC range from ≤0.015 to 0.03 mg/L, which was not affected by resistance to macrolides. This MIC range is consistent with that from other countries (MIC90: 0.002 mg/L) (Waites et al., 2017).
Lefamulin is the first semi-synthetic pleuromutilin antimicrobial agent approved for the treatment of CABP patients. Clinical trials have proved the excellent therapeutic effect of lefamulin. The MIC90 value of lefamulin was 0.5 μg/mL against the 50 strains of S. pneumoniae isolated from the patients in phase III clinical trial LEAP 1 (File et al., 2019) and 0.25 μg/mL against the 123 strains of S. pneumoniae isolated from the patients in clinical trial LEAP 2 (Alexander et al., 2019). The MIC90 against S. aureus isolates (10 and 13 strains) was 0.12–0.25 μg/mL. The post-treatment bacterial clearance rate was up to 100%. Research results at home and abroad have shown that lefamulin had similar antimicrobial activity against S. epidermidis and S. aureus (Paukner et al., 2013, 2019).
The above results support the excellent antimicrobial activity of lefamulin against CABP pathogens, especially antibiotic-resistant pathogens, such as PRSP, macrolide-resistant M. pneumoniae and MRSA. The major parameter driving efficacy for both S. aureus and S. pneumoniae is the 24h area under the drug concentration–time curve (AUC) over the MIC (24 h AUC/MIC). Lefamulin achieves rapid and predictable penetration into human tissues, with a mean 5.7-fold higher concentration in the pulmonary epithelial lining fluid compared with plasma. Percent probabilities of attaining the median AUCELF/MIC ratio targets associated with a 1-log10 CFU reduction from baseline by MIC were 97.0% at a MIC of 0.5 μg/mL for S. pneumoniae and 99.4% at a MIC of 0.25 μg/mL for S. aureus (Falcó et al., 2020). The unique mechanism of action, lack of cross resistance, good and broad coverage of respiratory pathogens regardless of resistance to other antimicrobial agent (Abbas et al., 2017; Lee and Jacobs, 2019) will surely make lefamulin a promising alternative treatment option in Chinese patients with CABP, especially those caused by PRSP, MRSA, or macrolide-resistant M. pneumoniae.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study protocol was approved by the Ethics Committee of Huashan Hospital, Fudan University (Number: 2019-319).
Author Contributions
DZ and FH designed the study. SW, YZ, YG, and DY performed the experimental work. SW and YZ collected the data. FH analyzed the data. All authors read and approved the final manuscript, contributed to the article, and approved the submitted version.
Funding
This work was supported by Sinovant Sciences, Major Research and Development Project of Innovative Antibiotics, Ministry of Science and Technology of China (No. 2017ZX09304005), National Mega-project for Innovative Drugs (2019ZX09721001-006-004), and CHINET Antimicrobial Surveillance Network (grant number WI207259).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Abbas, M., Paul, M., and Huttner, A. (2017). New and improved? a review of novel antibiotics for Gram-positive bacteria. Clin. Microbiol. Infect 23, 697–703. doi: 10.1016/j.cmi.2017.06.010
Alexander, E., Goldberg, L., Das, A. F., Moran, G. J., Sandrock, C., Gasink, L. B., et al. (2019). Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA 2019 322, 1661–1671. doi: 10.1001/jama.2019.15468
CLSI (2011). Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas; Approved Guideline (M43-A). Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2018). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (M07). Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2019). Performance standards for antimicrobial susceptibility testing (M100-S29). Wayne, PA: Clinical and Laboratory Standards Institute.
Falcó, V., Burgos, J., and Almirante, B. (2020). An overview of lefamulin for the treatment of community acquired bacterial pneumonia. Expert Opin. Pharmacother. 21, 629–636. doi: 10.1080/14656566.2020.1714592
File, T. M., Goldberg, L., Das, A., Sweeney, C., Saviski, J., Gelone, S. P., et al. (2019). Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial. Clin. Infect. Dis. 69, 1856–1867. doi: 10.1093/cid/ciz090
Lee, Y. R., and Jacobs, K. L. (2019). Leave it to lefamulin: a Pleuromutilin treatment option in community-acquired bacterial pneumonia. Drugs 79, 1867–1876. doi: 10.1007/s40265-019-01219-5
Mendes, R. E., Paukner, S., Doyle, T. B., Gelone, S. P., Flamm, R. K., and Sader, H. S. (2019). Low prevalence of gram-positive isolates showing elevated lefamulin MIC results during the SENTRY surveillance program for 2015-2016 and characterization of resistance mechanisms. Antimicrob. Agents Chemother 63:e02158-18. doi: 10.1128/AAC.02158-18
Paukner, S., Gelone, S. P., Arends, S. J. R., Flamm, R. K., and Sader, H. S. (2019). Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015–2016). Antimicrob. Agents Chemother 63:e02161-18. doi: 10.1128/AAC.02161-18
Paukner, S., Sader, H. S., Ivezic-Schoenfeld, Z., and Jones, R. N. (2013). Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob. Agents Chemother 57, 4489–4495. doi: 10.1128/aac.00358-13
Rodvold, K. A. (2019). Introduction: lefamulin and pharmacokinetic/pharmacodynamics rationale to support the dose selection of lefamulin. J. Antimicrob. Chemother 74(Suppl. 3), i2–i4.
Veve, M. P., and Wagner, J. L. (2018). Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy 38, 935–946. doi: 10.1002/phar.2166
Waites, K. B., Crabb, D. M., Duffy, L. B., Jensen, J. S., Liu, Y., and Paukner, S. (2017). In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother 61:e02008-16. doi: 10.1128/AAC.02008-16
Keywords: lefamulin, antimicrobial susceptibility test, minimum inhibitory concentration, community-acquired bacterial pneumonia, Mycoplasma pneumoniae
Citation: Wu S, Zheng Y, Guo Y, Yin D, Zhu D and Hu F (2020) In vitro Activity of Lefamulin Against the Common Respiratory Pathogens Isolated From Mainland China During 2017–2019. Front. Microbiol. 11:578824. doi: 10.3389/fmicb.2020.578824
Received: 01 July 2020; Accepted: 27 August 2020;
Published: 16 September 2020.
Edited by:
Shaolin Wang, China Agricultural University, ChinaReviewed by:
Anusak Kerdsin, Kasetsart University, ThailandLászló Majoros, University of Debrecen, Hungary
Copyright © 2020 Wu, Zheng, Guo, Yin, Zhu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fupin Hu, hufupin@fudan.edu.cn
 Shi Wu
Shi Wu Yonggui Zheng
Yonggui Zheng Yan Guo
Yan Guo Dandan Yin
Dandan Yin Demei Zhu1,2
Demei Zhu1,2 Fupin Hu
Fupin Hu