Abstract
The adsorption and electrokinetic properties of hybrid silica materials composed of nickel and silicon oxides (NixOy-SiO2), characterized by different contents of nickel oxide (from 0.5 to 3 mmol/g SiO2), were examined. These solids were also modified by poly(vinyl alcohol) to change their surface characteristics. The polymer is non-toxic and very well soluble in water. Due to incomplete hydrolysis of the polymer acetate groups, its macromolecules become negatively charged. The limited range of studied pH (6–10) resulted from high solubility of nickel oxide at more acidic pH values. The spectrophotometric, surface charge and electrophoretic measurements indicated that PVA exhibits higher adsorption affinity for the surfaces of mixed oxide with a larger content of nickel in its structure. Moreover, the presence of polymeric layers on the solid surface influences considerably the structure of electrical double layer formed at the mixed oxide-aqueous solution interface.
Similar content being viewed by others
Introduction
Nickel oxides belong to the group of transition metal compounds that exhibit semiconductor properties. Due to their anodic electrochromism [1], excellent thermal and electrochemical stability, unique optical properties these materials find a wide practical application. The most important of them are: catalysts (strong ability to absorb hydrogen up to 17 times the volume of the metal itself), battery cathodes, solar cells, gas sensors, electrochromic films and magnetic materials [2,3,4,5,6].
In the scientific literature much attention is paid to the synthesis and properties of nickel oxides whose particles are characterized by the nanosize [7,8,9,10]. The nanosized crystalline metal oxides show very good adsorption properties (because of their large surface areas, high concentration of active surface centers and surface defects presence) in relation to a large variety of molecules (starting with small ones—such as metal ions through those with the medium size—dyes, pesticides and amino acids, ending with large macromolecules—such as polymers) [11,12,13,14].
To obtain even better properties of oxide materials the synthesis of mixed systems was developed. There are a few methods of mixed oxides synthesis, among others, the most common are the following techniques: pyrogenic, sol–gel and chemical vapour deposition (CVD) [15,16,17]. The last one involves deposition of the second oxide layer from the vapour phase on the earlier prepared nanoparticles of the carrier oxide. As a result, the core–shell structure was formed. The deposition can be also performed from the liquid phase using various solvents, such as water and hexane. In such a way oxides of Mg, Mn, Ni, Cu, and Zn were deposited on the fumed silica [18]. At higher contents of the deposited oxide the growth of separate second phase on the carrier oxide surface (only a small fraction of the carrier oxide surface interacts with the deposited oxide) is possible.
Liu et al. synthesized mesostructured nickel silicate using nickel sulfate, CTAB cationic surfactant and sodium silicate [19]. The silica supported nickel oxide fine particles were also synthesized through sol–gel derived Ni–Al layered double hydroxide (LDH) and coated over the honeycomb ceramic pre-forms by the dip-coating technique [20]. Moreover, the silica-coated nickel oxides with the core–shell nanostructure using different concentrations of surfactant were prepared and characterized regarding of their shape, size, and phase structure [21]. Kondrashova et al. obtained mesoporous NiO–SiO2 silica-matrix composites with various nickel oxide concentrations by oxide cocondensation under hydrothermal synthesis conditions in the presence of cetyltrimethylammonium bromide as a template and (2-cyanoethyl) triethoxysilane as an organosubstituted trialkoxysilane additive [22].
The silica supported nickel oxide materials can be mainly applied in the metal-supported catalysis. The previous studies indicated that the presence of Ni particles and unreduced Ni ions within a silica matrix improves the stability and activity of the Ni-SiO2 catalysts in relation to in the carbon dioxide reforming of methane [23]. Ni-impregnated nickel over mesoporous silica with different Ni contents was synthesized and used for benzylation of benzene showing both high activity and high selectivity [24]. The nickel oxide-silica mixed systems can also find application as adsorbents in wastewaters treatment and medicine due to their antibacterial activity [25, 26].
Using the CVD method the NixOy-SiO2 mixed oxides with different contents of NixOy were prepared and applied in the present study. To modify their surface properties the poly(vinyl alcohol)—PVA layers were formed on the solid particles as a result of the adsorption process. The effects of nickel oxide content in the mixed silica materials, solution pH and polymer concentration were examined. The adsorption mechanism of PVA macromolecules on NixOy-SiO2 was explained based on the obtained changes in the solid surface charge density and zeta potential of mixed oxide particles in the polymer presence. Due to the solubility of nickel oxide below pH 6, experimental studies were carried out in the 6–10 pH range.
The PVA was used as an adsorbate due to its specific properties and also to get to know the surface characteristics of mixed oxides. The poly(vinyl alcohol) is classified as a non-ionic polymer (its –OH groups do not dissociate). However, due to the incomplete hydrolysis of acetate groups in the process of its production from poly(vinyl acetate), PVA macromolecules become negatively charged. The detailed mechanism of charge formation is discussed in the next section. These unique properties of PVA containing two types of functional groups leads to the increase of possible types of interactions with the solid surface. Besides, the applied polyalcohol is well soluble in water, biocompatible with human tissues and characterized by good biodegradability. It promotes its wide application in in the production of adhesives, coatings, pharmaceuticals, paints, paper, oils, fibers and hydrogels [27, 28].
In this paper a series of new silica based nickel oxide materials were obtained and modified with polymeric layers of poly(vinyl alcohol) with slight anionic character. Such NixOy-SiO2/PVA composites have not been described in the literature so far. The adsorption results were compared with those obtained for the analogous mixed oxides which were examined previously, that is MgxOy-SiO2 and ZnxOy-SiO2 [29]. It is worth noting that the coverage of mixed oxide particles (characterized by a quite narrow pH range in which no dissolution occurs) with a polymeric film considerably extends the pH range in which the oxide is stable) [30]. It is very important from the point of view of practical use of these composites as the range of their applications is considerably expanded compared to the acid–base properties of real systems.
Experimental
Materials
Four mixed oxides composed of nickel and silicon oxides (NixOy-SiO2) were used as adsorbents in the experiments. The solids were characterized by different contents of metal oxide, i.e. 0.5; 1; 2 or 3 mmol/g SiO2. They were denoted with the symbols: 05Ni-SiO2; 1Ni-SiO2; 2Ni-SiO2 and 3Ni-SiO2, respectively. All adsorbents were prepared in the Institute of Surface Chemistry of the National Academy of Sciences of Ukraine in Kiev.
The samples were synthesized in several stages. In the first stage, a homogeneous dispersion of silica was prepared in an aqueous solution of nickel acetate using the propeller stirrer “EUROSTAR power-b” at 500 rpm. The ratio of reagents in the aqueous dispersions was 0.5, 1.0. 2.0 and 3.0 mmol of nickel acetate per gram of A-380 silica. The second stage involved drying the dispersion in a 4–7 mm thick layer at 130 °C for 5 h. Next, the xerogel was ground in a porcelain mortar and sieved through a sieve with 0.5 mm cell. In the last stage of the synthesis, the powders were calcined at 600 °C in air for 2 h [31].
Thermal-oxidative degradation of adsorbed nickel acetate leads to the formation of the NiO/SiO2 system. In all samples, nickel oxide crystallites are formed with an average size of 14 nm [31]. At the same time, a part of nickel oxide is X-ray amorphous. For the samples 05Ni/SiO2, 1Ni/SiO2, 2Ni/SiO2 and 3Ni/SiO2, the degree of crystallinity is defined as 63, 64, 73 and 78%, respectively [31]. However, nickel oxide does not form a continuous layer on the surface of silica particles. The absence of a continuous shell of nickel oxide on the surface of SiO2 particles is confirmed by the SEM images presented in Fig. S1. The SEM images were obtained for solid samples characterized by different contents of metal oxide and was measured using high resolution scanning electron microscope Quanta 3D FEG (FEI, Field Electron and Ion Co.). Moreover, the elemental composition of applied adsorbents (excluding hydrogen) were determined using EDS (Energy Dispersive X-ray Spectroscopy) spectrometer (EDAX) which is the part of microscope system. The obtained data are presented in Table 1 and they confirmed the increase of Ni content in the surface layer of examined mixed oxides.
The specific surface area (SBET) of mixed oxides was determined using the low-temperature nitrogen adsorption–desorption method (ASAP 2420 analyzer, Micrometrics) and BET calculations [32]. The pore volume (Vp) was determined from the adsorption data at the relative pressure p/p0 ~ 0.98–0.99 [33]. The pore size distributions (and also contributions of micropores (Vmicro and Smicro), mesopores (Vmeso and Smeso) and macropores (Vmacro and Smacro)) were calculated using a self-consistent regularization (SCR) procedure under non-negativity condition (f V ≥ 0 at any pore radius R) at a fixed regularization parameter α = 0.01 using a complex model of slit-like and cylindrical pores [34]. The obtained results are presented in Table 2.
The mean particle diameter (d) of mixed oxides was measured using the apparatus Mastersizer 2000 (Malvern Instruments). The “wet” adapter Hydro 2000μP (aqueous suspension is sprayed via a rotary atomizer) was applied. The obtained d values (calculated by volume) are as follows: for 05 Ni-SiO2—110 nm, 1 Ni-SiO2—191 nm, 2 Ni-SiO2—255 nm and 3 Ni-SiO2—561 nm. It suggests, that solid particles with the highest content of metal atoms show the greatest tendency to aggregation.
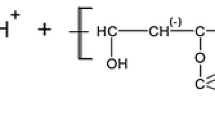
Poly(vinyl alcohol)—PVA with the molecular weight of 100,000 Da was used as the adsorbate. Besides the hydroxyl groups of nonionic character this macromolecular compound contains also acetate ones (as a product of incomplete hydrolysis of poly(vinyl acetate)). The monomer of poly(vinyl alcohol)—vinyl alcohol is very unstable and immediately undergoes isomerization to acetaldehyde and thus this polymer is obtained from poly(vinyl acetate). The degree of PVA hydrolysis was 86% and thus 14% of the acetate groups (-OCOCH3) is present in the polymeric macromolecules. The C–H bonds in α position in relation to the acetate groups possess acidic properties. The proton from the –CH2-segment, adjacent to that containing acetate groups, undergoes dissociation. The formed negative charge can be also concentrated on carbonyl oxygen of the acetate group due to the resonant structure creation. In this way the acetate groups in the PVA macromolecules gain a negative charge and are the source of a slight negative charge of PVA chains [29, 35].
All measurements were performed at 25 °C in the pH range 6–10. The NaCl solution with the concentration of 0.01 mol/dm3 was used as a supporting electrolyte. The limited range of studied pH values results from the solubility of nickel oxide at more acidic pH values.
Methods
The static method based on the polymer concentration decrease in the solution after the adsorption process was applied for determination of PVA adsorbed amounts. The PVA reaction with H3BO3 and I2 solutions [36] was used for this purpose. The green colour of polymeric solution with different intensities (depending on the PVA concentration) was obtained and the corresponding absorbance was measured spectrophotometrically at the wavelength 682 nm (UV/VIS spectrophotometer Cary 100, Varian). At the beginning the calibration curve was prepared (for the polymer concentrations changing in the range 3–200 ppm).
To determine the adsorbed amounts of PVA on the mixed oxide surface four PVA solutions with the concentration in the range 50–400 ppm were prepared. Then 0.02 g of the solid was added to each of them. The volume of polymer solution used in these tests was 10 cm3. The adsorption process was carried out under the conditions of continuous shaking (shaker Unimax 1010 (Heidolph) with the attached heating module (type Incubator 1000) for approx. 24 h and at three pH values: 6, 9 and 10. The pHs of examined systems were constantly checked and maintained on the constant level during the adsorption process. Next the samples were centrifuged (microcentrifuge (MPW Med. Instruments)) and clear solutions were subjected to further analysis. After the adsorption process the PVA concentration was determined using the above described procedure.
The polymer adsorption kinetics measurements were performed in two selected systems, namely 05 Ni-SiO2 or 3 Ni-SiO2. The processes were carried at pH 10 and at initial PVA concentration 200 ppm (using 0.02 g of the solid per 10 cm3 of the solution). The polymer adsorbed amounts were determined after 15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h and 6 h.
The desorption tests were performed at pH 10 at polymer initial concentration equal to 200 ppm in the systems containing 05 Ni-SiO2 or 3 Ni-SiO2. As desorbing agents HCl and NaOH solutions with concentration 0.05 mol/dm3 were used.
The potentiometric titration [37] was performed using a set consisting of: thermostated Teflon vessel, glass and calomel electrodes (Beckman Instruments), pH-meter PHM 240 (Radiometer), laboratory mixers, thermostat RE 204 (Lauda), automatic microburette Dosimat 765 (Metrohm) and computer. At the beginning 50 cm3 of the supporting electrolyte solution in the thermostatted vessel was prepared. Then this solution was titrated with NaOH (0.1 mol/dm3) in the pH range 6–11. As a result, the electrolyte curve (reference curve), showing the changes of pH versus the volume of the added base, was obtained. The analogous potentiometric titration was performed in the systems containing 0.1 g of NixOy-SiO2 and NaCl or NaCl + PVA solutions (at the polymer concentration 100 ppm). The solid surface charge density (σ0) calculations were done using the software “titr_v3” authored by Prof. W. Janusz and the following equation:
where: cb—base concentration, F—Faraday constant, m—solid mass in the suspension, S—specific surface area of the solid, ΔV—the difference in the volume of base which must be added to bring the pH of suspension and electrolyte to the specified value.
The zeta potential (ζ) of the mixed oxide particles was determined in the systems with and without poly(vinyl alcohol) using Zetasizer Nano ZS (Malvern Instruments). The suspensions were prepared adding 0.07 g of the solid to 150 cm3 of the supporting electrolyte or polymer solution (with the concentration 100 ppm). After the suspension sonication (3 min) applying an ultrasonicator XL 2020 (Misonix), the solution was divided into 6 parts, each of 20 cm3 volume. Then the appropriate pH value (5, 6, 7, 8, 9 and 10 ± 0.1) was adjusted using a pH-meter (Beckman Instruments). In the case of systems with PVA the polymer was added after the process of the solid suspension sonication. Electrophoretic mobility of solid particles dispersed in the liquid medium was measured using the dip cell (five repetitions of measurements for each sample). The zeta potential was calculated with the special computer program using the Henry equation [38]:
where: Ue—electrophoretic mobility, ε—dielectric constant, ε0—electric permeability of vacuum, ζ—zeta potential, η—viscosity, f(κa)—Henry function.
For NaCl with concentration 1 × 10−2 mol/dm3 κ−1 ≈ 3.04 nm [38]. The obtained κa values are as follows: for 05 Ni-SiO2—18.1, for 1Ni-SiO2—31.4, for 2 Ni-SiO2—41.9 and for 3 Ni-SiO2—92.3.
Results and Discussion
As can be seen in Table 2 the NixOy-SiO2 mixed oxides are characterized by an insignificantly smaller specific surface area in comparison to the pure silica. Moreover, the higher the Ni content in their structure is, the lower value the solid specific surface area assumes. All oxides do not contain macropores. The contribution of mesopores is large and the micropores content is small. The most noticeable specific surface area and volume of micropores were obtained for 1 Ni-SiO2.
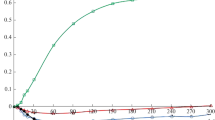
Figure 1 presents the exemplary adsorption isotherms of poly(vinyl alcohol) on the surface of 05 Ni-SiO2 mixed oxide obtained at three examined pH values (6, 9 and 10). The analogous dependencies were observed for all examined mixed oxides differing in the nickel content. The greatest adsorption level of the polymer was obtained at pH 10 whereas the lowest one at pH 9. On the other hand, the intermediate PVA adsorbed amount was observed at pH 6.
To discuss these dependencies the point of zero charges (pzc) of the examined oxides should be analyzed. Their values change from 8.38 (for 05 Ni-SiO2) to 8.97 (for 3 Ni-SiO2). The pHpzc for oxides and hydroxides of single nickel compounds changes in the wide range from 6.4 to 11.5 whereas that for SiO2 is in the range 1.7–3.5 [39]. This suggests that the surface of mixed oxides is mainly covered with the hydroxyl groups connected with Ni atoms and the silanol groups (containing Si atoms) are much less numerous.
The lowest PVA adsorption at pH 9 is probably caused by the fact that such pH value is very close to pHpzc of the examined mixed oxides. Under such conditions the solid surface is neutral (the number of positively (–SiOH2+, –Si–O–NiOH2+) and negatively (–SiO−, –Si–O–NiO−) charged surface groups is the same) and affinity of the polymeric chains for the solid surface groups is the lowest. The adsorption of poly(vinyl alcohol) increases at pH 6 at which solid surface is positively charged and electrostatic attractions with the negatively charged chains take place. The adsorbed PVA macromolecules conformation is rather flat and for this reason the polymeric layer packing is not large. The highest adsorption level of poly(vinyl alcohol) is achieved at pH 10. Under such conditions slight repulsions between the negatively charged adsorbent and the adsorbate take place. Besides these unfavourable forces the PVA adsorbed amount is more than three times larger than that in the solution of pH 6. This can be due to the most developed conformation of adsorbed chains towards the liquid phase which ensures large packing of the polymeric film. Under electrostatic repulsion conditions, the direct contact between adsorbent and adsorbate through train structures of adsorbed macromolecules is inconsiderable. The conformation of polymeric chains at the interface is rich in long loop and tail structures (ensuring high extension towards the liquid phase) and, as a result, many of them are adsorbed on the unit of the solid surface. This leads to increase of the PVA adsorption in the examined systems. Moreover, besides the electrostatic forces, formation of hydrogen bonds between the polymeric chains and the adsorbent surface is also possible [40]. Both types of polymer functional groups. In turn, among three types of solid surface groups (neutral –OH, positively charged –OH2+ and negatively charged-O−) the neutral –OH ones have the largest share in the polymer adsorption process, due to their greatest number [41]. Such phenomenon predominates mainly at pH 10 at which, despite the electrostatic repulsions between the solid surface and the PVA macromolecules, the polymer adsorption is the highest.
Additionally, the literature reports suggest [42] that in the strong basic solutions the silica support may undergo slight dissolution. In such a case, the positively charged active centers could be formed on the solid particles resulting in the remarkable growth of the negatively charged polymer chains attraction. As a consequence, the poly(vinyl alcohol) adsorption considerably increases.
As can be seen on Fig. 2 the PVA adsorption equilibria was reached after 5 h in the 05Ni-SiO2 system and after 4 h in the 3Ni-SiO2 system. The shorter equilibration time for a solid with higher Ni content is probably due to the stronger adsorption of the polymer on its surface.
The desorption tests performed under not vary “aggressive” conditions (using HCl and NaOH solutions with concentration 0.05 mol/dm3) indicated that adsorption layers of PVA adsorbed at pH 10 are rather stable (Table 3). In the case of mixed oxide with the highest Ni content the polymer desorption is inconsiderable (it not exceed 10%). Thus, the NixOy-SiO2/PVA composites can be successfully used in specific application areas.
The data presented in Fig. 3 indicate that the PVA adsorbed amounts increase with the increasing nickel content in the structure of mixed oxide. This is equivalent to an increase of the number of surface connections of –Si–O-Ni type. This suggests that the functional groups of the polymer show a greater affinity for the solid surface groups containing the metal atom but not for the silanol ones. The similar tendency was observed in the other mixed oxide systems (CuxOy-SiO2, MnxOy-SiO2, MgxOy-SiO2 and ZnxOy-SiO2) dispersed in the aqueous solution of poly(vinyl alcohol) [29, 43, 44].
The increase of the polymer initial concentration results in its greater adsorption on the NixOy-SiO2 surface (Fig. S2). Such behaviour is typical of the polymeric macromolecules characterized by a certain polydispersity index [45]. In such a case the polymeric sample contains macromolecules differing in the molecular weight in a certain range. When the polymer concentration in solution is small, all chains (with different molecular weights) are adsorbed on the solid surface. The increase of polymer concentration results in binding the PVA macromolecules with higher molecular weights (which was characterized by the greater free energy of adsorption than that of the polymeric chains with lower molecular weight). As a consequence, the PVA chains with the higher molecular weights show the greater affinity for the solid surface and the polymer adsorption grows.
The potentiometric titration results are presented in Fig. 4. They show the effects of Ni content in the mixed oxide structure (Fig. 4a) and PVA adsorption (Fig. 4b) on the solid surface charge density (σ0), as well as the values of pHpzc points characteristic of the examined systems (Fig. S3). As can be seen in Fig. 4 the value of pH corresponding with the solid zero charge increases when the nickel content in the mixed oxide also increases. The pHpzc increases by the value 0.59 for the examined hybrid silica materials (Fig. S3). This indicates that the greater number of -Si–O-Ni type surface groups (for NixOy-SiO2 containing higher contents of nickel—1, 2 and 3 mmol NixOy/g SiO2) causes rise in the solid surface acidity. In such a case the greater number of positively charged surface groups –Si–O–NiOH2+ are present at specific pH values (but not exceeding pHpzc). The changes in location of points of zero charge for the mixed oxides containing higher contents of NixOy are minimal (from 8.82 for 1 Ni–SiO2 to 8.97 for 3 Ni–SiO2). Additionally, the dependencies of the solid surface charge density versus the solution pH practically overlap for these systems.
The presence of poly(vinyl alcohol) adsorption layers on the surface of solid particles causes noticeable changes in σ0. In the case of 05 Ni–SiO2 mixed oxide the increase of solid surface charge density in the PVA presence was observed in the whole range of studied pH (in comparison to the analogous system without the polymer). On the other hand, for the suspension containing 3 Ni-SiO2 the decrease of σ0 was obtained (Fig. 4b)—the similar tendency occurs for the systems with 1 Ni–SiO2 and 2 Ni–SiO2 mixed oxides. These differences are probably caused by various conformations assumed by the adsorbed polymeric chains at the solid–liquid interface. The lower adsorption of poly(vinyl alcohol) on 05 Ni-SiO2 results in a flatter conformation of PVA macromolecules in the adsorption layer. In such situation the greater number of negatively charged acetate groups of the polymer can directly interact with the mixed oxide surface groups, which leads to the creation of the additional number of positively charged surface groups (–SiOH2+PVA−, –Si–O–NiOH2+PVA−)—the σ0 value increases. The increase in the polymer adsorbed amounts (occurring for the mixed oxide systems containing higher Ni contents) results in denser packing of PVA adsorption layer due to specific conformation of macromolecules which is more extended towards the solution. In such a case only a few polymeric segments (containing the ionized acetate groups) are directly bound with the solid surface. Their vast majority are located in the by-surface layer of the solution in the tail and loop structures of adsorbed chains causing a decrease of the solid surface charge [46].
The dependencies presenting the changes in the zeta (ζ) potential of mixed oxide particles as a function of solution pH have a completely different course (Fig. 5). This parameter characterizes the charge located in the slipping plane area formed around the solid particles. As can be seen in Fig. 5a the electrokinetic potential for the systems without the polymer assumes negative values in the whole range of studied pH and the extrapolated pHiep (iep—isoelectric point) is smaller than 5. The overlapping of electrical double layers (edl) formed in the adsorbent pores can be a main reason for the discrepancies between the values of pHpzc and pHiep [47]. Moreover, with the increasing content of nickel in the hybrid silica material, the zeta potential of solid particles assumes more negative values.
The electrokinetic behaviour of mixed oxide suspensions containing poly(vinyl alcohol) is completely different as indicated by the data presented in Fig. 5b. The values of ζ potential increase significantly for the NixOy-SiO2/PVA systems in comparison to the analogous suspensions without the polymer.
Similarly to solid surface charge density (described above), in suspensions containing ionic polymer there is no simple dependence between polymer adsorption and zeta potential of solid particles covered with polymeric layers. Changes in the zeta potential of solid particles in the ionic polymer presence are very complex and they are the result of three effects:
-
presence of negatively charged functional groups of the adsorbed polymer in the area of diffusion layer,
-
slipping plane shift caused by the macromolecules bound to the adsorbent surface,
-
displacement of the counter-ions located in the Stern layer due to adsorption of the polymer–desorption of supporting electrolyte ions from the solid surface; these ions are pushed out from the surface layer and can locate in the diffusion part of edl (electrical double layer) affecting the zeta potential of the adsorbent.
Differences in the PVA adsorbed amount obtained for mixed oxides characterized by increasing content of Ni atoms result in situation in which the above effects have various contributions to the final value of the zeta potential. As a result, their mutual addition may lead to the similar values of the electrokinetic potential.
The obtained changes in the examined systems can be mainly caused by the presence of electric charges in the adsorbed PVA macromolecules and the change in the slipping plane position caused by polymer adsorption. The changes in the ionic composition of the slipping plane area due to macromolecules binding and supporting electrolyte ions removal can be also of significant importance [48].
The comparison of the adsorbed amounts of poly(vinyl alcohol) on the surfaces of MexOy-SiO2 mixed oxides (Me: Ni, Zn, Mg) differing in the contents of Me obtained at pH 6 is presented in Fig. S4 [29]. All these oxides exhibit similar solubility in the aqueous solution depending on its pH. The greatest adsorption level of poly(vinyl alcohol) is achieved in the case of MgxOy-SiO2. For the metal content 3 mmol MexOy/g SiO2 it is over twelve times larger than that in the MgxOy-SiO2 containing system. Analyzing the textural parameters of these three solids containing different metal atoms, it should be concluded that they are similar. Thus, this factor minimally influenced the PVA adsorbed amount. In turn, MexOy-SiO2 mixed oxides (Me: Ni, Zn, Mg) differ with acid–base properties of their surfaces, which is reflected in the values of pHpzc points as well as sign and magnitude of solid surface charge. MgxOy-SiO2 was characterized by the highest value of pHpzc among the examined oxides. It is equal to about 10.5. For this reason at pH 6, at which the PVA adsorption is compared in Fig. S4, this oxide is endowed with the highest positive charge. As a result, the electrostatic adsorbent-adsorbate attraction in such a case is the greatest and the polymer adsorption reaches the highest level.
Conclusions
The increase of nickel content in the NixOy-SiO2 mixed oxide in the range 0.5–3 mmol/g SiO2 results in the noticeable increase of poly(vinyl alcohol)—PVA adsorption affinity for the solid surface. At pH 10 the PVA adsorbed amount increases from 0.12 mg/m2 (on the 05 Ni-SiO2 surface) to 0.16 mg/m2 (on the 3 Ni-SiO2 surface). This suggests that the polymeric segments are preferentially bound with the –Si–O–Ni surface groups (compared to the –Si–OH ones). The highest adsorption level of poly(vinyl alcohol) is observed at pH 10. Besides unfavourable electrostatic conditions at such pH value, the hydrogen bonds between the polymeric chains and the adsorbent surface are formed. The presence of poly(vinyl alcohol) adsorption layers on the surface of NixOy-SiO2 particles causes noticeable changes in the surface charge density and the zeta potential of the examined suspensions. It was shown that modification of NixOy-SiO2 mixed oxide surface with PVA changes its electrokinetic properties and leads to obtaining new silica based nickel materials with a great application potential.
References
R. Wen, C. G. Granqvist, and G. A. Niklasson (2015). Adv. Funct. Mater. 25, 3359.
G. Wang, Y. Ling, X. Lu, T. Zhai, F. Qian, Y. Tong, and Y. Li (2013). Nanoscale. 5, 4129.
X. Y. Deng and Z. Chen (2004). Mater. Lett. 58, 276.
O. E. Fayemi, A. S. Adekunle, and E. E. Ebenso (2016). J. Nanomat. ID 9614897, 12 pages.
A. Diallo, K. Kaviyarasu, S. Ndiaye, B. M. Mothudi, A. Ishaq, V. Rajendran, and M. Maaza (2018). Green Chem. Lett. Rev. 11, 166.
Z. Liu, A. Zhu, F. Cai, L. M. Tao, Y. Zhou, Z. Zhao, Q. Chen, Y. B. Cheng, and H. Zhou (2017). J. Mater. Chem. A 5, 6597.
M. El-Kemary, N. Nagy, and I. El-Mehasseb (2013). Mat. Sci. Semicond. Process. 16, 1747.
Y. Bahari Molla Mahaleh, S. K. Sadrnezhaad, and D. Hosseini (2008). J. Nanomat. ID 470595, 4 pages.
N. N. M. Zorkipli, N. H. M. Kaus, and A. A. Mohamad (2016). Procedia Chem. 19, 626.
C. T. Meneses, W. H. Flores, F. Garcia, and J. M. Sasaki (2007). J. Nanopart. Res. 9, 501.
M. Wiśniewska, I. Ostolska, K. Szewczuk-Karpisz, S. Chibowski, K. Terpiłowski, V. I. Zarko, and V. M. Gun’ko (2015). J. Nanopart. Res. 17, 12 (14 pages).
M. Wawrzkiewicz, M. Wiśniewska, V. M. Gun’ko, and V. I. Zarko (2015). Powder Technol. 278, 306.
A. M. Mahmoud, F. A. Ibrahim, S. A. Shaban, and N. A. Youssef (2015). Egypt J. Petrol. 24, 27.
S. M. Dehaghi, B. Rahmanifar, A. M. Moradi, and P. A. Azar (2014). J. Saudi Chem. Soci. 18, 348.
V. M. Gun’ko, Y. M. Nychiporuk, V. Zarko, E. V. Goncharuk, O. A. Mishchuk, R. Leboda, J. Skubiszewska-Zieba, E. Skwarek, W. Janusz, G. R. Yurchenko, V. D. Osovskii, Y. G. Ptushinskii, V. V. Turov, P. P. Gorbik, J. P. Blitz, and K. Gude (2007). App. Surf. Sci. 253, 3215.
V. M. Gun’ko, V. I. Zarko, V. V. Turov, R. Leboda, E. Chibowski, L. Holysz, E. M. Pakhlov, E. F. Voronin, V. V. Dudnik, and Y. I. Gornikov (1998). J. Colloid Interf. Sci. 198, 141.
F. Ciesielczyk, M. Przybysz, J. Zdarta, A. Piasecki, D. Paukszta, and T. Jesionowski (2014). J. Sol-Gel Sci. Tech. 71, 501.
V. M. Gun’ko, J. P. Blitz, B. Bandaranayake, E. M. Pakhlov, V. I. Zarko, I. Ya Sulym, K. S. Kulyk, M. V. Galaburda, V. M. Bogatyrev, O. I. Oranska, M. V. Borysenko, R. Eboda, J. Skubiszewska-Zięba, and W. Janusz (2012). Appl. Surf. Sci. 258, 6288.
X. Liu, C.-M. Chun, I. A. Aksay, and W. H. Shih (2000). Ind. Eng. Chem. Res. 39, 684.
M. Mishra, M. R. Das, and R. Goswamee (2010). J. Sol-Gel Sci. Tech. 54, 57.
R. G. Chaudhary, J. A. Tanna, A. Mondal, N. V. Gandhare, and H. D. Juneja (2017). J. Chin. Adv. Mater. Soc. 5, 103.
N. B. Kondrashova, I. V. Val’tsifer, V. N. Strel’nikov, V Ya Mitrofanov, and S. A. Uporov (2016). Inorg. Mater. 52, 909.
W. Cai, L. Ye, L. Zhang, Y. Ren, B. Yue, X. Chen, and H. He (2014). Materials 7, 2340.
M. Laribi, K. Bachari, R. Chebout, and M. Touati (2012). J. Assoc. Arab Univ. Basic Appl. Sci. 12, 42.
F. Motahari, M. R. Mozdianfard, and M. Salavati-Niasari (2015). Proc. Saf. Environ. Prot. 93, 282.
K. Harish, R. Renu, and S. S. Kumar (2011). Res. J. Chem. Sci. 1, 42.
V. G. Kadajji and G. V. Betageri (2011). Polym. 3, 1972.
A. Karimi and M. Navidbakhsh (2014). Mater. Technol. 29, 90.
M. Wiśniewska, P. Nowicki, V. M. Bogatyrov, A. Nosal-Wiercińska, and R. Pietrzak (2016). Colloids Surf. A 492, 12.
Ł. Klapiszewski, T. J. Szalaty, A. Kubiak, A. Skrzypczak, A. Dobrowolska, K. Czaczyk, and T. Jesionowski (2019). J. Mol. Liq. 274, 370.
V. M. Bogatyrov, L. I. Borysenko, O. I. Oranska, V. M. Gun’ko, R. Leboda, and J. Skubiszewska-Zięba (2010). Surface 2, 178.
S. Brunauer, P. H. Emmett, and E. Teller (1938). J. Am. Chem. Soc. 60, 309.
A. S. J. Gregg, and K. S. W. Sing (1982). Academic Press, London.
V. M. Gun’ko and S. V. Mikhalovsky (2004). Carbon. 42, 843.
P. Mastalerz Organic Chemistry (Chemistry Office, Wroclaw, 1998).
M. M. Zwick (1965). J. Appl. Polym. Sci. 9, 2393.
W. Janusz, E. Skwarek, V. I. Zarko, and V. M. Gun’ko (2007). Physicochem. Prob. Min. Proc. 41, 2015.
R. J. Hunter Zeta Potential in Colloid Science (Academic Press, New York, 1981).
M. Kosmulski (2016). Adv. Colloid Interf. Sci. 238, 1.
B. Kasprzyk-Hordern (2004). Adv. Colloid Interf. Sci. 110, 19.
S. Chibowski, M. Paszkiewicz, and M. Krupa (2000). Powder Tech. 107, 251.
E. R. Cook, and B. Batchelor (1996) in: Advances in porous media, Chapter 4, Elsevier.
M. Wiśniewska, V. Bogatyrov, K. Szewczuk-Karpisz, I. Ostolska, and K. Terpiłowski (2015). Appl. Surf. Sci. 356, 905.
M. Wiśniewska, V. Bogatyrov, I. Ostolska, K. Szewczuk-Karpisz, K. Terpiłowski, and A. Nosal-Wiercińska (2016). Adsorption. 22, 417.
R. P. Sear (1999). J. Chem. Phys. 111, 2255.
M. Wiśniewska (2010). Mater. Lett. 64, 1611.
E. Skwarek (2015). Ads. Sci. Tech. 33, 575.
M. Wiśniewska (2011). Colloid Polym. Sci. 289, 341.
Funding
There are no sources of financial funding and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving in Human Participants and Animals
The research did not involve human participants and/or animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wiśniewska, M., Chibowski, S., Urban, T. et al. Modification of Surface Properties of Colloidal Suspensions of NixOy-SiO2 Mixed Oxides with Different Ni Contents by the Adsorption Layers of Poly(Vinyl Alcohol). J Clust Sci 32, 1213–1221 (2021). https://doi.org/10.1007/s10876-020-01885-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01885-6