Abstract
Self-other sensory attribution is necessary to realize feedback control because the self-attribution of sensations can drive feedback control. Some studies have suggested that self-other attribution is realized by the integration of both sensorimotor cues, including internal prediction and/or sensory feedback, and cognitive cues, such as knowledge or thought. However, in motor control, it remains unclear whether and how cognitive cues affect self-other attribution. In a feedback-control task, this study manipulated the movements (sensorimotor cue) and appearances (cognitive cue) of the cursor provided as visual feedback on participants’ sinusoidal movement. Participants were required to make a self-other attribution regarding whether the cursor’s movement reflected their actual movement without being confused by the cursor’s appearance. Experiments 1 and 2 showed that participants made illusory self-other attributions within feedback control based on cursor appearance only when the information on cursor movement was reduced by causing the cursor to flicker at 8 Hz. However, in Experiment 3, in which the cursor flickering at 4 Hz reduced the information on cursor movement to a level too low for conscious self-other attribution, cursor appearance was not utilized. Our findings suggest that the effects of cognitive cues on self-other attribution are determined by the cue integration strategy selected for the given situation.
Similar content being viewed by others
Introduction
When people control their own movements based on sensory feedback, they typically feel that they are generating and controlling their own movements. This feeling has been referred to as the sense of agency (Gallagher, 2000; Haggard, 2017; Haggard & Chambon, 2012). Importantly, agency can drive feedback control through the self-attribution of sensations. This was shown in a study by Nielsen (1963): when participants performed a drawing task with visual feedback, the visual feedback of their actual movement was replaced with a fake movement, without their knowing. However, participants continued to respond to the fake visual feedback without noticing the deception. These results indicated that participants try to control movements if they attribute them to themselves even if they are not in fact self-induced (i.e., illusory feedback control). Therefore, we must determine self-other sensory attribution to realize accurate feedback control (Asai, 2015; Asai & Tanno, 2013; Nielsen, 1963; Toyomura & Omori, 2005). Studies have proposed that self-other attribution is realized through the integration of agency cues from different sources (Moore, 2016; Moore & Fletcher, 2012; Synofzik, Vosgerau, & Lindner, 2009; Synofzik, Vosgerau, & Voss, 2013). These cues can be categorized into sensorimotor and cognitive cues (e.g., Synofzik, Vosgerau, & Newen, 2008).
With regard to the former, studies have shown that self-other attribution depends on sensorimotor cues such as internal prediction (Blakemore, Wolpert, & Frith, 1998; Tsakiris, Haggard, Franck, Mainy, & Sirigu, 2005), proprioception (Balslev, Cole, & Miall, 2007; Farrer, Franck, Paillard, & Jeannerod, 2003), and vision (Fourneret & Jeannerod, 1998; Preston & Newport, 2014). The effects of sensorimotor cues may be based on the comparator model in the computational theory of motor control (Blakemore, Wolpert, & Frith, 2002; Frith, Blakemore, & Wolpert, 2000). According to the comparator model, the brain predicts sensory feedback (Wolpert, Ghahramani, & Jordan, 1995; Wolpert & Kawato, 1998; Wolpert, Miall, & Kawato, 1998). When the prediction matches the sensory feedback, the sensation is attributed to the self; that is, registration of agency occurs (Blakemore, Wolpert, & Frith, 2000b; Sato & Yasuda, 2005). From this perspective, Asai (2015) examined the role of sensorimotor cues in the dynamic relationship between self-other attribution and feedback control. Participants traced a sine wave (i.e., a sinusoidal movement) with a pen and received visual feedback in the form of a cursor’s movement on a monitor. When participants received the cursor representing a prerecorded movement of their own that was spatiotemporally similar to participants’ actual movement (i.e., low levels of prediction error; Wolpert & Flanagan, 2001), they misattributed the fake movement to themselves and tried to control the cursor. This result supports the finding that feedback control can be driven by the self-attribution of sensations and suggests that self-other attribution is based on the sensorimotor system of motor control (Asai, 2015; Weiss, Tsakiris, Haggard, & Schütz-Bosbach, 2014), which computes prediction error by comparing internal prediction and sensory feedback (i.e., sensorimotor cues).
In contrast, other evidence has shown that self-other attribution is modulated by cognitive cues such as thoughts, knowledge, or beliefs (Aarts, Custers, & Wegner, 2005; Desantis, Roussel, & Waszak, 2011; Linser & Goschke, 2007; van der Weiden, Aarts, & Ruys, 2011; Wegner, Sparrow, & Winerman, 2004; Wegner & Wheatley, 1999). For example, in a study in which participants were shown a preview of a subsequent action, even though they had not in fact performed that action, they experienced an illusory sense of control (i.e., self-attribution of the action; Wegner et al., 2004). Moreover, beliefs or experiences related to causality modulate intentional binding (Desantis et al., 2011; Dogge, Schaap, Custers, Wegner, & Aarts, 2012). In other words, the perceived interval between actions and outcomes is used as an implicit measure of the sense of agency (Haggard, Clark, & Kalogeras, 2002). As an example of this, intentional binding increased when participants were told that they had caused an outcome. The effects of these cognitive cues can be explained in the light of three principles of apparent mental causation: priority, consistency, and exclusivity (Wegner, 2003; Wegner et al., 2004; Wegner & Wheatley, 1999). According to these principles, the sense of agency may be enhanced when a cognitive cue is introduced just before an action (priority), is consistent with the action (consistency), and is not accompanied by alternative causes of the action (exclusivity). This notion has been supported by previous studies showing that the sense of agency is strengthened by the prime regarding the actions’ effects (Aarts et al., 2005; Linser & Goschke, 2007; Sato, 2009; Wenke, Fleming, & Haggard, 2010). As the prime works when it precedes the action and is consistent with the action’s effect, the priming effect is based on priority and consistency principles (Wegner, 2003).
Thus, both sensorimotor and cognitive cues may contribute to self-other attribution; however, the relationship between these cues remains unclear in terms of their effect on self-other attribution. Recently, this relationship has been mentioned in the framework of optimal cue integration (Moore, 2016; Synofzik et al., 2009, 2013; Vosgerau & Synofzik, 2012). In this framework, the weight of a certain cue on the registration of agency is determined according to its relative reliability in a given situation. For example, if an agency cue includes a great deal of noise mixed with the information, the reliability of this cue is low and, accordingly, other information cues are given more weight. Namely, the relative contribution of a certain type of cue to the registration of agency might depend on the context. Some studies have supported the cue integration hypothesis in terms of implicit or explicit indicators such as intentional binding (Moore, Wegner, & Haggard, 2009; Wolpe, Haggard, Siebner, & Rowe, 2013) or explicit agency judgment (Gentsch, Kathmann, & Schütz-Bosbach, 2012; Wen, Yamashita, & Asama, 2015). According to cue integration theory, self-other attribution might depend on sensorimotor cues in the context of motor control because they directly contribute to realizing feedback control, while cognitive cues do not do so. In fact, no studies so far have examined whether and how cognitive cues affect self-other attribution within feedback control. The cue integration strategy in motor control (i.e., the relationship between sensorimotor and cognitive cues for the registration of agency in motor control) remains unclear. Therefore, the present study focused on self-other attribution in motor control.
The present study examined the effects of cognitive cues on self-other attribution within feedback control. To investigate self-other attribution in terms of motor control, the basic procedure followed that of Asai’s (2015) study. The cursor movement was defined as the sensorimotor cue and utilized in the computation of prediction error between participants’ actual movements and the cursor’s movements. To examine the effects of cognitive cues, the prime paradigm was applied to the feedback-control task. The appearance of the cursor with two patterns (circle and asterisk) was defined as a cognitive cue. This cue functioned as a priming effect through prior experience. Specifically, participants experienced self-other attributions based on cursor appearance prior to the main part of the movement task because the cursor appearance corresponded to the cursor’s movement in the prior actions. The present study investigated the cues (i.e., movement or appearance of the cursor) that participants utilized for self-other attribution within feedback control after being primed with cursor appearance.
Furthermore, we explored the relationship between sensorimotor and cognitive cues through three experimental situations in which the amount of available visual feedback was manipulated. In Experiment 1, the amount of cursor movement information (the sensorimotor cue) was sufficient to enable participants to rely on it. Based on the cue integration theory, we expected that only the sensorimotor cue would be utilized for self-other attribution within feedback control. However, in Experiments 2 and 3, the amount of visual feedback (i.e., cursor movement information) was reduced by causing the cursor to flicker (Asai, 2015). In such cases, we hypothesized that the flicker would cause a relationship between the sensorimotor and cognitive cues to alter according to the availability and reliability of the sensorimotor cue. Specifically, self-other attribution was expected to be modulated by cognitive cues only in a situation where sensorimotor cues were insufficiently reliable. The results would shed light on how cognitive cues affect self-other attribution within feedback control.
Experiment 1
Experiment 1 examined the effects of cognitive cues on self-other attribution within feedback control. The basic procedure followed that of Asai’s (2015) study wherein participants traced a sine wave composed of five cycles and received visual feedback. To examine the effects of cognitive cues, a prime paradigm was applied to this task. Given that the sensorimotor cue might be given more weighting than the cognitive cue in the context of motor control, we hypothesized that participants would utilize only cursor movement (sensorimotor cue) for self-other attribution within feedback control, even if they were primed with cursor appearance (cognitive cue).
Method
Participants
To determine sample size, we conducted a power analysis (alpha = .05 and Cohen’s d = .45) based on the planned analysis that included a main effect and a three-way interaction. This analysis was conducted using PANGEA (Power ANalysis for GEneral Anova designs), which enables the computation of a sample size for a multifactor analysis of variance (ANOVA; Westfall, 2016). Although Cohen’s d did not match the effect size (ηp2) for the present study, we used the default value (i.e., Cohen’s d = .45) in this analysis because Westfall (2016) recommends using it as is. Concerning the three-way interaction, this analysis indicated that having ten participants was sufficient to achieve power of .80. Concerning the main effect, this analysis indicated that having 21 participants was necessary to achieve power of .80. In Experiment 1, 21 healthy right-handed volunteers (mean age = 26.7 years, SD = 7.7) were recruited. This experiment was conducted with the approval of the Ethics Committee of Kio University (no. H30-15). Each participant provided written informed consent.
Apparatus
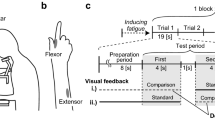
An LED monitor (LCD-EA223WM-B3; NEC) was set parallel to and 20 cm above a digitizing tablet (Intuos4 PTK-1240/K0; Wacom), which displayed a sine wave (target line) composed of five cycles (Fig. 1; Asai, 2015). A cycle was defined as five periods between the negative peaks of the sine wave. The size of the plotting area of the monitor was almost the same as that of the input area of the digitizer (488 × 305 mm). The refresh rate of the monitor was 60 Hz. The experiment was programmed using Hot Soup Processor 3.4 (Onion Software).
Feedback control task
The basic procedure followed that of Asai’s (2015) study. Participants manipulated a pen device on the digitizer to trace the target line (i.e., sinusoidal movement) as accurately as possible and received visual feedback in the form of the cursor’s movement on the monitor (Fig. 1). During the preparation time of 5 s, participants placed the pen at the start position (on the left side of the target line). When the computer started to count from zero, participants started moving the pen toward the peak of the first cycle. In each trial, participants attempted to trace the target line toward the goal position (right-hand side of the target line) while timing their movements so that the timing of the pen tip reaching the peak or trough of the sine wave matched the counting from 1 s to 10 s. Movement error – the vertical distance between the pen position and the target line – served as an index of motor performance. For the statistical analyses, the average movement error for each cycle was calculated. Participants became familiar with the required movement and the device by training before the main experiment.
Self-other attribution in motor performance
To examine the effects of the sensorimotor cue, the cursor movement reflected either participants’ actual movement or a prerecorded movement; these were designated as the SELF and FAKE conditions, respectively. In the SELF condition, participants received visual feedback on their actual movements in the form of the cursor’s movement and were required to control the cursor by altering their pen movements when necessary. In the FAKE condition, participants were shown a prerecorded cursor movement over which they had no control and were required to ignore the cursor to keep the pen tip on track. In this condition, participants had to trace the target line using only proprioception without any visual feedback. The prerecorded movements were participants’ own movements that had been secretly recorded during the practice session. During the practice session, participants performed 40 trials of tracing the target line while watching a square-shaped cursor, which provided visual feedback of their actual movements. The first ten trials were not used as the prerecorded movements because participants were not yet familiar with the procedure. Therefore, the prerecorded movements were randomly chosen from the last 30 practice trials.
In this paradigm (Asai, 2015), when the cursor deviated from the target line, if participants attributed the cursor movement to themselves, they would try to manipulate the pen to compensate for the deviated trajectory of the cursor. Simultaneously, if the cursor represented participants’ actual movements, they would succeed in the compensatory movement (i.e., less movement error) because they could control its cursor. However, if the cursor in fact represented a prerecorded movement over which participants had no control, movement error would be greater because of the illusory compensatory movement. To realize accurate tracing movements (i.e., less movement error), participants first needed to form a correct self-other attribution regarding the cursor movement based on online spatiotemporal consistency (i.e., prediction error) between their actual movements and that of the cursor. The cursor was masked for the first and last 0.5 s to prevent participants from distinguishing between SELF and FAKE movements based on when they started and finished each movement.
First half of the feedback control task
To examine the effects of the cognitive cue, we incorporated cursor appearances into Asai’s paradigm and set the sine wave to separate into Cycles 1 and 2 (first half) and Cycles 4 and 5 (second half). Cycles 1 and 2 were set to prime cursor appearance to participants through prior action experiences. Regarding the prior action, CONTROLLED and IGNORED conditions were designed (Fig. 2). In the CONTROLLED condition, participants were shown a circle-shaped cursor that followed their actual movements. Therefore, participants were required to trace the target line by controlling the cursor (prior control). In the IGNORED condition, participants were shown an asterisk-shaped cursor that followed a prerecorded movement. In this condition, participants were required to ignore the cursor and to trace the target line using proprioception alone while ignoring the visual information from the cursor. The sizes of the circle- and asterisk-shaped cursors were almost the same (9-mm diameter). Before the experiment, participants were instructed as follows: “A circle-shaped cursor represents your actual movement, whereas an asterisk-shaped cursor represents PC-controlled movement. Therefore, in the first half of the sine wave, you must distinguish between self- and PC-controlled movements by referring to the appearance of the cursor. Specifically, you have to control the circle-shaped cursor and ignore the asterisk-shaped cursor.”
Schematic representation of the experimental design. Cursor movement and appearance were defined as sensorimotor cue and cognitive cue, respectively. In Cycles 1 and 2 (first half), the cursor movement types (SELF and FAKE) corresponded to the appearances (CIRCLE and ASTERISK) of the cursor. Participants experienced self-other attributions based on the cursor appearance in the first half. In Cycles 4 and 5 (second half), the correspondence between movement type and cursor appearance changed in some trials: there were four conditions (SELF-CIRCLE, SELF-ASTERISK, FAKE-CIRCLE, and FAKE-ASTERISK) in each of the two first-half conditions (CONTROLLED and IGNORED). Participants had to make self-other attributions based on online spatiotemporal consistency between their actual movements and the cursor movements, without allowing themselves to make self-other attributions with reference to cursor appearance
Cursor appearance (cognitive cue) functioned as a priming effect in Cycles 4 and 5 through prior action experiences (prior control or ignore) of self-other attribution based on cursor appearance. In particular, controlling the cursor (i.e., prior control) was expected to make the priming effects stronger than when ignoring it because participants had to switch their motor strategy from visual feedback (i.e., the asterisk-shaped cursor) to proprioception after ignoring, which indicated that the prior ignore did not have the priority principle for the cognitive cue (also see the General discussion). A preliminary experiment in which the correspondences were reversed so that the circle- and asterisk-shaped cursors corresponded to the fake and self-movements, respectively, confirmed that the cursor appearance itself did not have a specific effect as the cognitive cue.
Mask component
In Cycle 3, participants traced the target line using proprioception alone without visual feedback because the cursor was masked in all conditions. This masking cycle was necessary to prevent participants from being confused by a sudden change in the cursor movements and appearances, or from merely continuing to control (or ignore) the cursor without consciously making a self-other attribution. Although the movement error in Cycle 3 was not of interest and was thus not analyzed, it served as the baseline for the movement error in Cycles 4 and 5.
Second half of the feedback control task
In Cycles 4 and 5, the cursor was masked for the first 0.5 s of Cycle 4 to conceal the moment when Cycle 3 ended and Cycle 4 began; afterward, participants were shown the cursor again. The correspondence between movement type and cursor appearance may have changed from Cycles 1 and 2. Regarding the change in visual feedback, there were four conditions: SELF-CIRCLE, SELF-ASTERISK, FAKE-CIRCLE, and FAKE-ASTERISK (Fig. 2). Therefore, information about cursor appearance was not useful to distinguish cursor movements in Cycles 4 and 5. Participants were instructed: “The cursor movements and appearances may change from the first half to the second half. Therefore, you have to ignore the cursor appearances in the second half.” This instruction was necessary to prevent participants from retaining the causally false belief that a circle-shaped cursor always indicated their own movements, which was crucial to enable this study to examine the effects of the cognitive cues in self-other distinction. Participants were also instructed: “In the second half of the sine wave, you have to judge whether the cursor is representing your own movement or a PC-controlled movement based on the spatiotemporal consistency between your pen movement and the cursor movement. When you feel the cursor movement is your own movement, you can control it. When you feel the cursor movement is not your own movement but is a PC-controlled movement, you have to ignore it and trace the sine wave without visual feedback.”
In summary, participants were required to make self-other attributions based on online spatiotemporal consistency (i.e., prediction error) between their actual movements and cursor movements, without allowing themselves to make self-other attributions based on cursor appearance.
Subjective self-other judgment
After each trial, participants reported their confidence in their subjective self-other judgment on a 9-point scale (Asai, 2016) ranging from very confident that it was a self-movement (9) to very confident that it was a fake movement (1). This judgment was reported by pressing the corresponding number key. To quantify participants’ incorrect responses (subjective misattributions) in each condition, we calculated the differences between the correct score (i.e., 9 for the SELF conditions and 1 for the FAKE conditions) and the actual mean score. Specifically, we subtracted the mean score from 9 for the SELF conditions and subtracted 1 from the mean score for the FAKE conditions; thus, incorrect response scores were positive.
Baseline conditions
There were two baseline conditions: one for CONTROLLED and one for IGNORED. In both conditions, participants were required to trace the target line without visual feedback in Cycles 4 and 5. In the CONTROLLED-baseline condition, the procedure in Cycles 1 and 2 was the same as that in the CONTROLLED condition (i.e., the circle-shaped cursor followed participants’ actual movements). In the IGNORE-baseline condition, no cursor was presented even in Cycles 1 and 2; this enabled us to confirm that participants were successfully ignoring asterisk-shaped cursors presenting fake movements in Cycles 1 and 2. If participants ignored the asterisk-shaped cursors in Cycles 1 and 2 as instructed, the movement error would be almost the same as that in the IGNORE-baseline condition. Furthermore, if participants controlled the circle-shaped cursors (i.e., self-movements) in the CONTROLLED condition, the movement error would be less than it was in the IGNORE-baseline condition. Regarding the movement errors in the first half, therefore, the comparison with the IGNORE-baseline condition enabled us to confirm whether participants were properly controlling the circle-shaped cursor and ignoring the asterisk-shaped cursor.
As there were four conditions (SELF-CIRCLE, SELF-ASTERISK, FAKE-CIRCLE, and FAKE-ASTERISK) in each of the two first half conditions (CONTROLLED and IGNORED), this study comprised eight visual feedback conditions and two baseline conditions. As each condition was tested 12 times in a random order, there were 120 trials in this study. Before the main experiment, participants performed ten trials (one trial of each condition) to become familiar with the experimental procedure.
Results and discussion
Since the self-attribution of visual feedback can drive feedback control (Asai, 2015; Nielsen, 1963), if participants attributed the movements of all circle-shaped cursors to themselves, they would try to control all circle-shaped cursors – even those that were in fact following prerecorded movements. In that case (i.e., trying to control the prerecorded movement), because of the illusory feedback control, movement error would be greater than it would be when they ignored asterisk-shaped cursors following prerecorded movements (also see Self-other attribution in motor performance section in Method). Moreover, if participants tried to ignore all asterisk-shaped cursors, movement error would be greater when they were shown asterisk-shaped cursors following self-movements than when they were shown circle-shaped cursors following self-movements. Therefore, if we confirmed significant differences between the CIRCLE and ASTERISK conditions, it would indicate that participants made self-other attributions based on cursor appearance (i.e., the effects of the cognitive cue). Further, if we confirmed that there were significant differences between the SELF and FAKE conditions, it would indicate that participants made self-other attributions based on cursor movement (i.e., the effects of the sensorimotor cue).
Although the primary outcome was movement error in the second half (Cycles 4 and 5), we first analyzed participants’ confidence in their self-other judgment and their movement error in the first half (Cycles 1 and 2), which was related to the quality of the cognitive cues.
Subjective self-other judgment
Regarding the incorrect responses (subjective misattributions), a 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK) revealed no significant three-way interaction, F(1, 20) = .15, p = .71, ηp2 = .007, no significant main effect of appearance, F(1, 20) = .18, p = .67, ηp2 = .009, and a significant main effect of movement, F(1, 20) = 82.80, p < .001, ηp2 = .81, indicating that misattributions were significantly larger in the FAKE condition than in the SELF condition (Fig. 3). In addition, considering the small effect sizes regarding appearance and the large effect size for movement, these results indicate that participants made subjective self-other judgments based on the spatiotemporal consistency between their own movements and those of the cursor (sensorimotor cue), but not based on cursor appearance (cognitive cue).
Incorrect responses (subjective misattributions) on the subjective self-other judgment in Experiments 1, 2, and 3. In the SELF conditions, incorrect responses indicate alternate attribution of a self-directed movement; i.e., participants shown a cursor reflecting their actual movements stated that it showed another’s movement. In the FAKE conditions, incorrect responses indicate self-attribution of a prerecorded movement; i.e., participants shown a cursor presenting a prerecorded movement stated that it showed their own actual movement. Error bars indicate standard errors
Movement error in the first half
Regarding the four conditions (SELF-CIRCLE, SELF-ASTERISK, FAKE-CIRCLE, and FAKE-ASTERISK) in each of the two first half conditions (CONTROLLED and IGNORED), movement errors in the first half were analyzed to investigate whether participants properly controlled the circle-shaped cursor and ignored the asterisk-shaped cursor. This analysis was conducted by comparing the CONTROLLED, IGNORED, and IGNORE-baseline conditions. In each of the two first half conditions, because the procedures among the four conditions were the same and the differences in movement errors among these conditions were not of interest, the average of the movement errors among these conditions and between Cycles 1 and 2 was calculated (Fig. 4). A one-way within-participants ANOVA revealed a significant difference among the CONTROLLED, IGNORED, and IGNORE-baseline conditions, F(2, 40) = 43.17, p < .001, ηp2 = .68. A post hoc test (Shaffer’s modified sequentially rejective Bonferroni procedure) revealed that the movement error in the CONTROLLED condition was significantly smaller than those in the IGNORED condition, t(20) = 9.15, p < .001, r = .90, and the IGNORE-baseline condition, t(20) = 7.17, p < .001, r = .85, and there was no significant difference between the IGNORED and IGNORE-baseline conditions, t(20) = .78, p =.45, r = .18. These results in the first half showed that participants properly controlled the circle-shaped cursor and ignored the asterisk-shaped cursor, suggesting that they based their self-other attributions on cursor appearance as instructed.
Mean movement errors between Cycles 1 and 2 for the CONTROLLED, IGNORED, and IGNORE-baseline conditions in Experiments 1, 2, and 3. The average of the movement errors among four conditions (SELF-CIRCLE, SELF-ASTERISK, FAKE-CIRCLE, and FAKE-ASTERISK) in each of the two first-half conditions (CONTROLLED and IGNORED) was calculated. In the IGNORE-baseline condition, participants received no cursor during their motor performance. In Experiments 2 and 3, the cursor was set to flicker at 8 and 4 Hz, respectively (i.e., the available information on cursor movement was reduced). Error bars indicate standard errors
Movement error in the second half
The differences in movement errors between each visual feedback condition and its corresponding baseline condition (CONTROLLED- or IGNORE-baseline condition) were calculated. Owing to the differences in movement error among all conditions for Cycle 3, it appeared possible that the movement errors in the second half may have been either underestimated or overestimated. To equalize these differences, the movement error for Cycle 3 was subtracted from that in each of the other cycles, so that the movement error in each condition was 0 at Cycle 3. The averages of the movement errors between Cycles 4 and 5 were submitted to a 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK).
This analysis revealed no significant three-way interaction, F(1, 20) = .048, p = .83, ηp2 = .002, no significant main effect of appearance, F(1, 20) = .001, p = .98, ηp2 = .00, and a significant main effect of movement, F(1, 20) = 209.47, p < .001, ηp2 = .91, indicating that the movement errors in the FAKE condition were significantly larger than those in the SELF condition (Fig. 5). Considering the small effect sizes regarding appearance and the large effect size for movement, these results indicate that participants used cursor movement (sensorimotor cue), but not cursor appearance (cognitive cue), to make self-other attributions. Even after participants had experienced prior control (i.e., CONTROLLED condition), no effects of the cognitive cues were observed. Some studies claimed that internal prediction is the most reliable agency cue in conditions in which sufficient information is available (Sato, 2009; Synofzik et al., 2013). In the current paradigm, the sensorimotor cue was necessary to enable comparison with participants’ internal predictions of the movement; therefore, the sensorimotor cue had a greater effect on self-other attribution, as hypothesized.
Movement errors in Cycles 4 and 5 in Experiment 1. The vertical axis shows the differences in movement error between the visual feedback conditions and baselines (no visual-feedback conditions). The movement error in Cycle 3 was subtracted from that in each of the other cycles. The differences between the CIRCLE and ASTERISK conditions indicated that participants made self-other attributions based on cursor appearance. The differences between the SELF and FAKE conditions indicated that participants made self-other attributions based on cursor movement. Error bars indicate standard errors
According to cue integration findings, the weight of a certain cue on the registration of agency depends on the reliability of any other available cues (Synofzik et al., 2013). If cue integration explains self-other attribution in motor control, the effects of cognitive cues should be observed in situations where sensorimotor cues are less reliable. According to Asai’s (2015) study, receiving less visual feedback makes it more difficult to calculate prediction error. For example, when the visual feedback was reduced by flickering the cursor at 8 Hz, participants made less compensatory movement, indicating that they made less illusory self-attribution of fake movements. Reduced visual feedback makes the computation of prediction error more difficult, thereby making the sensorimotor cue less reliable. In this situation, the decreased reliability of the sensorimotor cue could increase participants’ reliance on the cognitive cue, even in the context of motor control. Experiment 2 examined this hypothesis.
Experiment 2
In Experiment 2, the amount of available visual feedback was reduced by flickering the cursor at 8 Hz (Asai, 2015). As described in Asai’s (2015) study, this reduction in visual feedback makes the computation of prediction error more difficult. In this situation, in which the sensorimotor cue was less reliable, we hypothesized that participants would rely more heavily on the cognitive cue for self-other attribution, which would affect feedback control.
Method
Participants
The sample size was decided according to the rules laid down in Experiment 1. Twenty-one healthy right-handed volunteers (mean age = 26.9 years, SD = 8.8) were recruited. Each participant provided written informed consent.
Procedure
The cursor was set to flicker at 8 Hz throughout each trial (Asai, 2015). Except for this flickering, the procedure and analyses were the same as those in Experiment 1. A preliminary experiment confirmed that participants could recognize different cursor shapes when the cursor was flickering.
Results and discussion
Subjective self-other judgment
Regarding incorrect responses (subjective misattribution), a 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK) revealed no significant three-way interaction, F(1, 20) = .075, p = .79, ηp2 = .004, no significant main effect of appearance, F(1, 20) = .54, p = .47, ηp2 = .026, and a significant main effect of movement, F(1, 20) = 13.07, p = .002, ηp2 = .40, indicating that misattributions were significantly larger in the FAKE condition than in the SELF condition (Fig. 3). In addition, considering the small effect sizes regarding appearance and the large effect size for movement, these results indicate that cursor movement (sensorimotor cue), but not cursor appearance (cognitive cue), was still used for subjective self-other judgment even though the visual feedback was reduced by the cursor’s flickering.
Movement error in the first half
A one-way within-participants ANOVA revealed a significant difference among the CONTROLLED, IGNORED, and IGNORE-baseline conditions, F(2, 40) = 46.20, p < .001, ηp2 = .70 (Fig. 4). A post hoc test (Shaffer’s modified sequentially rejective Bonferroni procedure) revealed that the movement error in the CONTROLLED condition was significantly smaller than those in the IGNORED condition, t(20) = 9.71, p < .001, r = .91, and the IGNORE-baseline condition, t(20) = 8.29, p < .001, r = .89, and there was no significant difference between the IGNORED and IGNORE-baseline conditions, t(20) = .45, p =.66, r = .10. The results in the first half indicated that participants successfully controlled the circle-shaped cursor and ignored the asterisk-shaped cursor, which is consistent with the results of Experiment 1.
Movement error in the second half
A 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK) revealed a significant three-way interaction, F(1, 20) = 62.51, p < .001, ηp2 = .76, and main effect of movement, F(1, 20) = 51.29, p < .001, ηp2 = .72. In addition, considering that the effect size for the three-way interaction was clearly larger than that in Experiment 1 (ηp2 = .002), these results indicate that the flickering cursor altered the relationship between the sensorimotor and cognitive cues from that observed in Experiment 1, as hypothesized (Fig. 6).
Movement errors in Cycles 4 and 5 in Experiment 2. The vertical axis shows the differences in movement error between the visual feedback conditions and baselines (no visual-feedback conditions). The movement error in Cycle 3 was subtracted from that in each of the other cycles. The differences between the CIRCLE and ASTERISK conditions indicated that participants made self-other attributions based on cursor appearance. The differences between the SELF and FAKE conditions indicated that participants made self-other attributions based on cursor movement. Asterisks indicate significant simple effects in mean movement error between Cycles 4 and 5. Error bars indicate standard errors
Regarding the simple effects of the prior action, the movement errors were significantly larger in the CONTROLLED conditions than in the IGNORED conditions in the SELF-ASTERISK condition, F(1, 20) = 8.08, p = .010, ηp2 = .29, and in the FAKE-CIRCLE condition, F(1, 20) = 12.54, p = .002, ηp2 = .39. These results indicate that participants tried to control the circle-shaped cursor and ignore the asterisk-shaped cursor, regardless of the cursor movements, after they had controlled the circle-shaped cursor in the first half. Given that participants significantly ignored the asterisk-shaped cursor, these results suggest not that participants merely continued to control the circle-shaped cursor after the prior control, but that they made self-other attributions based on the correspondence between cursor movement and appearance. Therefore, the prior control might have affected the priming effects of the correspondences and thereby influenced self-other attribution in motor control.
Regarding the simple effects of cursor appearance, in the CONTROLLED condition, movement error was significantly larger in the SELF-ASTERISK condition than in the SELF-CIRCLE condition, F(1, 20) = 28.37, p < .001, ηp2 = .59, and movement error was significantly larger in the FAKE-CIRCLE condition than in the FAKE-ASTERISK condition, F(1, 20) = 19.64, p < .001, ηp2 = .50. These results indicate that self-other attribution within feedback control was affected by cursor appearance (cognitive cue). Conversely, in the IGNORED condition, there were no significant effects of cursor appearance in the SELF condition, F(1, 20) = 2.36, p = .14, ηp2 = .11, or in the FAKE condition, F(1, 20) = 1.57, p = .22, ηp2 = .073. If stronger attention to the flickering cursor had caused these results, the increased movement errors should also have been observed in the IGNORED conditions. However, the results did not show this. The effects of cursor appearance were induced only when participants had controlled the circle-shaped cursor in the first half. Therefore, these results indicate that the increase in movement errors (i.e., misattribution) was owing to the combination of prior control and cursor appearance in the condition where information on cursor movement was reduced through flickering.
In addition to the effects of the cognitive cue, the main effect and simple effects of movement (i.e., sensorimotor cues) were significant. Regarding the simple effects of movement, the movement errors were significantly larger in the FAKE conditions than in the SELF conditions in the CONTROLLED-CIRCLE condition, F(1, 20) = 82.20, p < .001, ηp2 = .80, in the CONTROLLED-ASTERISK condition, F(1, 20) = 19.87, p < .001, ηp2 = .50, in the IGNORED-CIRCLE condition, F(1, 20) = 22.48, p < .001, ηp2 = .53, and in the IGNORED-ASTERISK condition, F(1, 20) = 37.78, p < .001, ηp2 = .65. These results indicate that participants made self-other attributions based on cursor movement; however, they also used cursor appearance. The cognitive cue might have contributed to self-other attributions that were primarily based on the sensorimotor cue.
In Experiment 2, in addition to the main effect of the cursor movement, simple effects of prior control and cursor appearance were observed when the cursor was flickering at 8 Hz. These results indicate that the cognitive cues modulated feedback control based on the sensorimotor cue. The effects of cognitive cues are not independent in motor control; rather, they might work on the basis of sensorimotor cues. If so, cognitive cues would no longer be utilized if the amount of information on cursor movement (sensorimotor cue) decreased too low for self-other attribution. In Asai’s (2015) study, compensatory pen movement in response to a prerecorded cursor movement was not observed when the cursor was flickering at 4 Hz (i.e., when the information on cursor movement was further reduced), which suggests that this level of cursor movement information was too low to enable the computation of prediction error in motor control. In such a situation, compensating by using cognitive cues would not be reasonable for motor control because cognitive cues are not directly related to motor control. However, there is an alternative possibility – that participants used only cognitive cues instead of sensorimotor cues because the latter were reduced by flickering. In other words, self-other attribution could be based on a simple switch of attribution strategy from the sensorimotor cue to the cognitive cue. In this case, the less visual feedback is provided, the more cognitive cues are utilized. To investigate these possibilities, Experiment 3 was conducted.
Experiment 3
In Experiment 3, the amount of available visual feedback was reduced to a level lower than that in Experiment 2 (i.e., by flickering the cursor at 4 Hz), thereby making the computation of prediction error even more difficult (Asai, 2015). If self-other attribution in motor control is grounded within the sensorimotor cue, the effects of the cognitive cues would not be observed in this situation. This would indicate that cognitive cues are not utilized based on an attribution strategy switch from the sensorimotor cue to the cognitive cue, but rather are only used in a context-dependent strategy (i.e., in the context of motor control).
Method
Participants
The sample size was decided according to the rules laid down in Experiment 1. Twenty healthy right-handed volunteers (mean age = 26.6 years, SD = 7.8) were recruited. Each participant provided written informed consent.
Procedure
The cursor was set to flicker at 4 Hz throughout the trial (Asai, 2015). Except for flickering, the procedure and analyses were the same as those in Experiments 1 and 2. A preliminary experiment confirmed that participants could recognize the different shapes of the flickering cursor.
Results and discussion
Subjective self-other judgment
Regarding incorrect responses (subjective misattribution), a 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK) revealed no significant interactions or main effects, including the three-way interaction, F(1, 19) = .16, p = .70, ηp2 = .008, main effect of movement, F(1, 19) = .029, p = .87, ηp2 = .002, and main effect of appearance, F(1, 19) = .57, p = .46, ηp2 = .029 (Fig. 3). In addition, considering the small effect size for movement, this result indicates that it was difficult to subjectively detect the differences between SELF and FAKE movements, suggesting that cursor movement information (sensorimotor cue) was insufficient to correctly make conscious self-other attributions. This result is not consistent with that of Asai’s (2015) study, in which a significant difference was found between the SELF and FAKE conditions. This may be because participants had just two cycles wherein they received visual feedback, as opposed to the four cycles in Asai’s study. Because a greater number of cycles has advantages for detecting prediction errors, in a situation where the cursor was flickering at 4 Hz, less cycles (i.e., two cycles instead of four cycles) may have caused difficulty for participants to convince themselves that the cursor movement was self-directed even when it really was self-directed.
Movement error in the first half
A one-way within-participants ANOVA revealed a significant difference among the CONTROLLED, IGNORED, and IGNORE-baseline conditions, F(2, 38) = 26.93, p < .001, ηp2 = .59 (Fig. 4). A post hoc test (Shaffer’s modified sequentially rejective Bonferroni procedure) revealed that the movement error in the CONTROLLED condition was significantly smaller than those in the IGNORED condition, t(19) = 7.99, p < .001, r = .88, and the IGNORE-baseline condition, t(19) = 5.29, p < .001, r = .77, and there was no significant difference between the IGNORED and IGNORE-baseline conditions, t(19) = .52, p = .61, r = .12. The results in the first half indicate that participants controlled the circle-shaped cursor and ignored the asterisk-shaped cursor, as in Experiments 1 and 2.
Movement error in the second half
A 2 × 2 × 2 within-participants ANOVA with factors of prior action (CONTROLLED and IGNORED), movement (SELF and FAKE), and appearance (CIRCLE and ASTERISK) revealed no significant three-way interaction, F(1, 19) = .096, p = .76, ηp2 = .005, no significant main effect of appearance, F(1, 19) = .62, p = .44, ηp2 = .032, and a significant main effect of movement, F(1, 19) = 29.50, p < .001, ηp2 = .61, indicating that the movement errors were significantly larger in the FAKE condition than in the SELF condition (Fig. 7). This result indicates that participants controlled the cursor in the SELF movement conditions. Interestingly, the effect size for the three-way interaction was clearly smaller than that in Experiment 2 (ηp2 = .76); but it was similar to that in Experiment 1 (ηp2 = .002). In addition, considering the small effect size for the main effect of appearance, the results indicate that participants did not use cursor appearance to make self-other attributions. These results do not support the possibility that the cognitive cue is used instead of the reduced sensorimotor cue.
Movement errors in Cycles 4 and 5 in Experiment 3. The vertical axis shows the differences in movement error between the visual feedback conditions and baselines (no visual-feedback conditions). The movement error in Cycle 3 was subtracted from that in each of the other cycles. The differences between the CIRCLE and ASTERISK conditions indicated that participants made self-other attributions based on cursor appearance. The differences between the SELF and FAKE conditions indicated that participants made self-other attributions based on cursor movement. Error bars indicate standard errors
However, there is a possibility that the difficulty in controlling the flickering cursor in the first half weakened the effects of the prior control. Since the effects of the cognitive cues were linked with the prior control (i.e., interaction effects), the weakening of the effects of prior control might have diminished the effects of cognitive cues. According to the results in the first half, participants could control the flickering circle-shaped cursor; if participants had had difficulty controlling the flickering cursor, they would have made more movement errors than they had in Experiments 1 and 2. We therefore examined this possibility by comparing the movement errors in the first halves of the trials among all experiments.
Additional analysis
Regarding the movement errors in the first half of each experiment, there were no significant differences among the four conditions and between Cycles 1 and 2 in the CONTROLLED condition. Since these differences were not of interest in this analysis, the average among these conditions and between cycles was calculated. A one-way between-participants ANOVA with a factor of experiment (Experiments 1, 2, and 3) revealed that there were no significant differences among Experiments, F(2, 59) = .67, p = .52, ηp2 = .02. This result does not support the possibility that additional difficulty in controlling the circle-shaped cursor weakened the effects of the prior control in Experiment 3. In the first half, participants formed self-other attributions using a cursor appearance. This result therefore suggests that it is possible to realize feedback control of the cursor as long as self-other attribution is achieved, even when the cursor is flickering at 4 Hz.
To summarize, the results of Experiment 3 did not show that the less the sensorimotor cue was provided the more the cognitive cue was utilized; rather, they indicated that self-other attributions within feedback control were based on the sensorimotor cue even when the amount of available information on the cursor movement was further reduced. In motor control, the effects of cognitive cues might be limited to particular situations.
General discussion
This study examined the effects of cognitive cues on self-other attribution within feedback control. Furthermore, we explored the relationship between sensorimotor and cognitive cues in terms of cue integration through three experiments in which the amount of available visual feedback information was manipulated. Experiment 1 showed that participants utilized only the cursor movements for self-other attribution, suggesting that sensorimotor cues have the strongest effect on self-other attribution in motor control. In Experiment 2, the amount of available visual feedback (the sensorimotor cue) was reduced by setting the cursor to flicker at 8 Hz. In this situation, the cognitive cues confused feedback control through misattribution of the cursor’s movements. Conversely, significant misattributions owing to cognitive cues were not observed in the subjective self-other judgment. Finally, in Experiment 3, in which the cursor was set to flicker at 4 Hz, no effects of the cognitive cue were seen. These results might represent one of the cue integration strategies in self-other attribution within feedback control.
Explicit agency and its limitations in the current paradigm
In this study, motor performance (i.e., movement error) and subjective self-other judgment were adopted as the implicit and explicit measures of agency, respectively (Asai, 2015). However, our results showed significant misattributions owing to cognitive cues in motor performance, but not in subjective self-other judgment. This difference suggests that these processes might be based on different mechanisms (e.g., Dewey & Knoblich, 2014). Since participants were instructed to ignore cursor appearance in the second half (i.e., Cycles 4 and 5), they should have tried not to rely on cursor appearance as an agency cue in forming their conscious self-other attributions. Subjective judgment is susceptible to bias from other cognitive factors, such as causal knowledge or background information (Haggard, 2017; Synofzik et al., 2008, 2013; van der Weiden et al., 2011). Consequently, such information might have surpassed the effects of cursor appearance on subjective self-other judgment. Moreover, this subjective judgment included postdictive inference since participants gave their judgments after engaging in motor performance. The instructions they were given might have had different influences on these two measures. This limitation is inherent to this study design because it cannot reveal the time course of subjective self-other attribution (Asai, 2015). Concerning the implicit and explicit agency, the cue integration processes might indeed differ (Synofzik et al., 2013). However, we examined the effects of cognitive cues in terms of motor control. Further studies examining the cue integration process in each aspect of agency should be conducted.
Feedback control confused by cognitive cues
Although feedback control is linked to agency (e.g., Asai, 2015), the relationship between feedback control and cognitive agency cues has not been thoroughly examined. Nielsen’s (1963) classic study could be interpreted as having shown the effects of cognitive cues in feedback control. In this study, even if the visual feedback of participants’ own movement was replaced with a fake movement without them knowing, they continued to attribute the fake visual feedback to themselves and tried to control it without discovering the deception (see Introduction). Although this study has been interpreted to mean that feedback control is modulated through self-other attribution, it could also be interpreted to mean that feedback control was affected by the cognitive cue, such as the causally false belief “I am the author of the visual feedback.” Since the visual feedback was replaced in secret, it seems that this belief was based on the exclusivity principle proposed by Wegner and Wheatley (1999): there was no conspicuous alternative cause for the actions witnessed by participants. Since participants therefore exclusively believed that the visual feedback represented their own movements, it is unsurprising that they attributed the fake movements to themselves because the exclusivity principle is powerful (Wegner et al., 2004). In the present study, although cognitive cues affected feedback control in a particular situation, participants did not adopt the false belief that the visual feedback represented their own movements because they were told that cursor appearance was causally irrelevant to the origin of the cursor movement. Therefore, the cognitive cues in the present study should not have been based on the exclusivity principle.
In Experiment 2, in which the sensorimotor cue information was reduced, the effects of the cognitive cue were observed only when participants had experienced prior control. In the CONTROLLED condition, since the cursor appearance was actually utilized to realize feedback control in the first half (i.e., Cycles 1 and 2), the experience of prior control would have contributed to making the cognitive cue work as the prime. In the IGNORED condition, conversely, participants had to switch their motor strategy from visual feedback (i.e., asterisk-shaped cursor) to proprioception after making a self-other attribution based on cursor appearance. This prior action would have been considered a decision based not on the cursor appearance but rather on participants’ proprioception. The prior IGNORED condition trial, therefore, would not have had the priority principle for the cognitive cue. Consequently, the prior IGNORED condition trial may have diminished the priming effects of cognitive cues. These results suggest that the effects of cognitive cues were based on both priority and consistency principles.
Motor control, especially feedback control, generally has to be realized by using sensorimotor cues such as internal prediction and sensory feedback (e.g., Wolpert et al., 1995). Conversely, cognitive cues are not directly linked to feedback control. In the present study, however, cognitive cues had an impact on feedback control through self-other attribution. Even if participants’ actual movements did not match the cursor movements (i.e., error detection), when they attributed the cursor movements to themselves through cognitive cues, they tried to control the cursor (i.e., illusory feedback control). This supports the notion that feedback control is based not on error detection but rather on self-other attribution (e.g., Asai, 2015). Even cognitive cues that are based on priority and consistency principles without exclusivity could modulate self-other attribution in motor control. However, it should be considered that this effect may be limited to particular situations.
Cue integration strategy for motor control
As reported in Asai’s (2015) study, a flickering cursor makes the computation of prediction error more difficult. The resulting reduction in visual feedback makes this sensorimotor cue less reliable. In our study, the combined effects of the prior control and cognitive cues were observed only when the sensorimotor cue information was reduced by flickering the cursor at 8 Hz. The diminished reliability of the sensorimotor cue might explain why, in this situation, the cognitive cue can exert its effects. The effects of the cognitive cues were not observed, however, when the cursor was flickering at 4 Hz, suggesting that the relationship between the sensorimotor and cognitive cues is not a linear relationship in that the less reliable the sensorimotor cue is, the more weight the cognitive cue is given. In terms of motor control, when the sensorimotor cue is less reliable, cognitive cues might compensate for this missing information in self-other attribution, when the sensorimotor cue is so limited or unreliable that it cannot be used; however, cognitive cues might no longer be utilized to make self-other attributions. Since feedback control itself is based on sensorimotor cues such as sensory feedback, in such a situation, it would not be reasonable for cognitive cues to be utilized instead of the impaired sensorimotor cue to realize feedback control. In motor control, cognitive cues are not independent but they might work to support sensorimotor cues. The contribution of each agency cue to the formation of self-other attributions might be determined according to the context-dependent strategy (Synofzik et al., 2009, 2013).
The results partially disagreed with those of a previous study, which suggested that self-other attribution is based on the integration of agency cues (Moore et al., 2009). That study manipulated the prime corresponding to the effect of the subsequent voluntary or involuntary movement to modulate the sense of agency measured by intentional binding (i.e., the perceived interval between movement and effect). Consequently, the perceived intervals were modulated to a greater degree by the prime in involuntary movement; but some modulation was observed even in voluntary movement. This result indicates that the prime (i.e., the cognitive cue) could affect the sense of agency even in a situation where the internal signals (i.e., sensorimotor cues) were sufficiently available and reliable because of the voluntary nature of the movement. In the present study, however, the effects of cognitive cues were significant only when the sensorimotor cue was less reliable. This difference might be owing to the characteristics of the tasks adopted in these two studies: the intentional binding task in the previous study as opposed to the feedback-control task in our own. In the intentional binding task, participants are simply required to press a key, and it is not necessary to continuously control the movement. Moreover, some studies have suggested that intentional binding reflects causality but not necessarily agency or intentionality (Buehner, 2012; Dogge et al., 2012). Conversely, in the feedback-control task, participants were required to control a continuous movement by comparing their internal predictions with visual feedback; therefore, sensorimotor cues might be given more weight in the feedback-control task. This notion is consistent with the cue integration evidence that the weight of each agency cue depends on the given situation (Synofzik et al., 2009, 2013). Moreover, the sources of agency cues and priors should be considered. That is, if various studies adopt different cues and priors, they might yield different results (i.e., different cue integration strategies according to the context).
Cue integration may contribute to flexible agency in variable contexts (Synofzik et al., 2009, 2013). However, it does allow for the possibility that an impaired sensorimotor system could cause agency disruptions owing to the collapsed relationship between the sensorimotor and cognitive cues ( Moore, 2016; Moore & Fletcher, 2012; Synofzik et al., 2013). For example, patients with schizophrenia often have difficulty distinguishing their own actions and outcomes from those of others (Bulot, Thomas, & Delevoye-Turrell, 2007; Daprati et al., 1997; Franck et al., 2001; Hauser et al., 2011; van der Weiden, Prikken, & van Haren, 2015). Some studies have shown that internal prediction in schizophrenia is imprecise and that this was associated with frequent misattributions in patients with schizophrenia (Blakemore, Smith, Steel, Johnstone, & Frith, 2000a; Synofzik, Thier, Leube, Schlotterbeck, & Lindner, 2010; Voss et al., 2010). According to cue integration, their imprecise predictions could cause compensatory dependence on cognitive cues (e.g., Moore, 2016). Indeed, some studies have shown that patients with schizophrenia depend more on external cues than on internal prediction (Synofzik et al., 2010; Voss et al., 2010). Moreover, schizophrenia can cause motor abnormalities (e.g., Blyler, Maher, Manschreck, & Fenton, 1997), and it has been suspected that a disturbed agency based on an impaired sensorimotor system is involved in these motor abnormalities (Izawa, Asai, & Imamizu, 2016). From this perspective, cue integration in motor control leads to the prediction that patients with schizophrenia could take externally generated sensations into motor control through compensatory self-other attribution depending on additional cognitive cues. However, more evidence is needed before cue integration can be used to predict behaviors in daily life; it will be essential, for example, to reveal the differences in cue integration strategy between healthy people and patients with schizophrenia in terms of motor control. Furthermore, continuous studies should seek to uncover how this difference influences the movements of patients with schizophrenia.
In summary, the present study suggests that the effects of cognitive cues on self-other attribution within feedback control are determined by various cue integration strategies according to the given situation. In the context of motor control, self-other attribution may be based on sensorimotor cues linked to our movement itself. However, only when sensorimotor cues are less reliable – but still available – can cognitive cues be utilized for self-other attribution. Therefore, cognitive cues might have a compensatory function on self-other attribution that is grounded within sensorimotor cues.
References
Aarts, H., Custers, R., & Wegner, D. M. (2005). On the inference of personal authorship: Enhancing experienced agency by priming effect information. Consciousness and Cognition, 14(3), 439–458. doi: https://doi.org/10.1016/j.concog.2004.11.001
Asai, T. (2015). Feedback control of one’s own action: Self-other sensory attribution in motor control. Consciousness and Cognition, 38, 118–129. doi: https://doi.org/10.1016/j.concog.2015.11.002
Asai, T. (2016). Self is “other”, other is “self”: Poor self-other discriminability explains schizotypal twisted agency judgment. Psychiatry Research, 246, 593–600. doi: https://doi.org/10.1016/j.psychres.2016.10.082
Asai, T., & Tanno, Y. (2013). Why must we attribute our own action to ourselves? Auditory hallucination like-experiences as the results both from the explicit self-other attribution and implicit regulation in speech. Psychiatry Research, 207(3), 179–188. doi: https://doi.org/10.1016/j.psychres.2012.09.055
Balslev, D., Cole, J., & Miall, R. C. (2007). Proprioception contributes to the sense of agency during visual observation of hand movements: Evidence from temporal judgments of action. Journal of Cognitive Neuroscience, 19(9), 1535–1541. doi: https://doi.org/10.1162/jocn.2007.19.9.1535
Blakemore, S.-J., Smith, J., Steel, R., Johnstone, E. C., & Frith, C. D. (2000a). The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: Evidence for a breakdown in self-monitoring. Psychological Medicine, 30(5), 1131–1139.
Blakemore, S.-J., Wolpert, D., & Frith, C. (2000b). Why can’t you tickle yourself? Neuroreport, 11(11), R11–16.
Blakemore, S.-J., Wolpert, D. M., & Frith, C. D. (1998). Central cancellation of self-produced tickle sensation. Nature Neuroscience, 1(7), 635–640. doi: https://doi.org/10.1038/2870
Blakemore, S.-J., Wolpert, D. M., & Frith, C. D. (2002). Abnormalities in the awareness of action. Trends in Cognitive Sciences, 6(6), 237–242. doi: https://doi.org/10.1016/S1364-6613(02)01907-1
Blyler, C. R., Maher, B. A., Manschreck, T. C., & Fenton, W. S. (1997). Line drawing as a possible measure of lateralized motor performance in schizophrenia. Schizophrenia Research, 26(1), 15–23. doi: https://doi.org/10.1016/S0920-9964(97)00040-6
Buehner, M. J. (2012). Understanding the past, predicting the future: Causation, not intentional action, is the root of temporal binding. Psychological Science, 23(12), 1490–1497. doi: https://doi.org/10.1177/0956797612444612
Bulot, V., Thomas, P., & Delevoye-Turrell, Y. (2007). A pre-reflective indicator of an impaired sense of agency in patients with schizophrenia. Experimental Brain Research, 183(1), 115–126. doi: https://doi.org/10.1007/s00221-007-1027-8
Daprati, E., Franck, N., Georgieff, N., Proust, J., Pacherie, E., Dalery, J., & Jeannerod, M. (1997). Looking for the agent: An investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition, 65(1), 71–86. doi: https://doi.org/10.1016/S0010-0277(97)00039-5
Desantis, A., Roussel, C., & Waszak, F. (2011). On the influence of causal beliefs on the feeling of agency. Consciousness and Cognition, 20(4), 1211–1220. doi: https://doi.org/10.1016/j.concog.2011.02.012
Dewey, J. A., & Knoblich, G. (2014). Do implicit and explicit measures of the sense of agency measure the same thing? PLoS ONE, 9(10), e110118. doi: https://doi.org/10.1371/journal.pone.0110118
Dogge, M., Schaap, M., Custers, R., Wegner, D. M., & Aarts, H. (2012). When moving without volition: Implied self-causation enhances binding strength between involuntary actions and effects. Consciousness and Cognition, 21(1), 501–506. doi: https://doi.org/10.1016/j.concog.2011.10.014
Farrer, C., Franck, N., Paillard, J., & Jeannerod, M. (2003). The role of proprioception in action recognition. Consciousness and Cognition, 12(4), 609–619. doi: https://doi.org/10.1016/S1053-8100(03)00047-3
Fourneret, P., & Jeannerod, M. (1998). Limited conscious monitoring of motor performance in normal subjects. Neuropsychologia, 36(11), 1133–1140. doi: https://doi.org/10.1016/S0028-3932(98)00006-2
Franck, N., Farrer, C., Georgieff, N., Marie-Cardine, M., Daléry, J., d’Amato, T., & Jeannerod, M. (2001). Defective recognition of one’s own actions in patients with schizophrenia. The American Journal of Psychiatry, 158(3), 454–459. doi: https://doi.org/10.1176/appi.ajp.158.3.454
Frith, C. D., Blakemore, S.-J., & Wolpert, D. M. (2000). Abnormalities in the awareness and control of action. Philosophical Transactions of the Royal Society B, 355(1404), 1771–1788. doi: https://doi.org/10.1098/rstb.2000.0734
Gallagher, S. (2000). Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences, 4(1), 14–21.
Gentsch, A., Kathmann, N., & Schütz-Bosbach, S. (2012). Reliability of sensory predictions determines the experience of self-agency. Behavioural Brain Research, 228(2), 415–422. doi: https://doi.org/10.1016/j.bbr.2011.12.029
Haggard, P. (2017). Sense of agency in the human brain. Nature Reviews Neuroscience, 18(4), 196–207. doi: https://doi.org/10.1038/nrn.2017.14
Haggard, P., & Chambon, V. (2012). Sense of agency. Current Biology, 22(10), R390–R392. doi: https://doi.org/10.1016/j.cub.2012.02.040
Haggard, P., Clark, S., & Kalogeras, J. (2002). Voluntary action and conscious awareness. Nature Neuroscience, 5(4), 382–385. doi: https://doi.org/10.1038/nn827
Hauser, M., Knoblich, G., Repp, B. H., Lautenschlager, M., Gallinat, J., Heinz, A., & Voss, M. (2011). Altered sense of agency in schizophrenia and the putative psychotic prodrome. Psychiatry Research, 186(2), 170–176. doi: https://doi.org/10.1016/j.psychres.2010.08.003
Izawa, J., Asai, T., & Imamizu, H. (2016). Computational motor control as a window to understanding schizophrenia. Neuroscience Research, 104, 44–51. doi: https://doi.org/10.1016/j.neures.2015.11.004
Linser, K., & Goschke, T. (2007). Unconscious modulation of the conscious experience of voluntary control. Cognition, 104(3), 459–475. doi: https://doi.org/10.1016/j.cognition.2006.07.009
Moore, J. W. (2016). What is the sense of agency and why does it matter? Frontiers in Psychology, 7, 1272. doi: https://doi.org/10.3389/fpsyg.2016.01272
Moore, J. W., & Fletcher, P. C. (2012). Sense of agency in health and disease: A review of cue integration approaches. Consciousness and Cognition, 21(1), 59–68. doi: https://doi.org/10.1016/j.concog.2011.08.010
Moore, J. W., Wegner, D. M., & Haggard, P. (2009). Modulating the sense of agency with external cues. Consciousness and Cognition, 18(4), 1056–1064. doi: https://doi.org/10.1016/j.concog.2009.05.004
Nielsen, T. I. (1963). Volition: A new experimental approach. Scandinavian Journal of Psychology, 4(1), 225–230. doi: https://doi.org/10.1111/j.1467-9450.1963.tb01326.x
Preston, C., & Newport, R. (2014). Noisy visual feedback training impairs detection of self-generated movement error: Implications for anosognosia for hemiplegia. Frontiers in Human Neuroscience, 8. https://doi.org/10.3389/fnhum.2014.00456
Sato, A. (2009). Both motor prediction and conceptual congruency between preview and action-effect contribute to explicit judgment of agency. Cognition, 110(1), 74–83. doi: https://doi.org/10.1016/j.cognition.2008.10.011
Sato, A., & Yasuda, A. (2005). Illusion of sense of self-agency: Discrepancy between the predicted and actual sensory consequences of actions modulates the sense of self-agency, but not the sense of self-ownership. Cognition, 94(3), 241–255. doi: https://doi.org/10.1016/j.cognition.2004.04.003
Synofzik, M., Thier, P., Leube, D. T., Schlotterbeck, P., & Lindner, A. (2010). Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain, 133(1), 262–271. doi: https://doi.org/10.1093/brain/awp291
Synofzik, M., Vosgerau, G., & Lindner, A. (2009). Me or not me—An optimal integration of agency cues? Consciousness and Cognition, 18(4), 1065–1068. doi: https://doi.org/10.1016/j.concog.2009.07.007
Synofzik, M., Vosgerau, G., & Newen, A. (2008). Beyond the comparator model: A multifactorial two-step account of agency. Consciousness and Cognition, 17(1), 219–239. doi: https://doi.org/10.1016/j.concog.2007.03.010
Synofzik, M., Vosgerau, G., & Voss, M. (2013). The experience of agency: An interplay between prediction and postdiction. Frontiers in Psychology, 4, 127. doi: https://doi.org/10.3389/fpsyg.2013.00127
Toyomura, A., & Omori, T. (2005). Auditory feedback control during a sentence-reading task: Effect of other’s voice. Acoustical Science and Technology, 26(4), 358–361. doi: https://doi.org/10.1250/ast.26.358
Tsakiris, M., Haggard, P., Franck, N., Mainy, N., & Sirigu, A. (2005). A specific role for efferent information in self-recognition. Cognition, 96(3), 215–231. doi: https://doi.org/10.1016/j.cognition.2004.08.002
van der Weiden, A., Aarts, H., & Ruys, K. I. (2011). Prime and probability: Causal knowledge affects inferential and predictive effects on self-agency experiences. Consciousness and Cognition, 20(4), 1865–1871. doi: https://doi.org/10.1016/j.concog.2011.09.007
van der Weiden, A., Prikken, M., & van Haren, N. E. M. (2015). Self–other integration and distinction in schizophrenia: A theoretical analysis and a review of the evidence. Neuroscience & Biobehavioral Reviews, 57, 220–237. doi: https://doi.org/10.1016/j.neubiorev.2015.09.004
Vosgerau, G., & Synofzik, M. (2012). Weighting models and weighting factors. Consciousness and Cognition, 21(1), 55–58. doi: https://doi.org/10.1016/j.concog.2011.09.016
Voss, M., Moore, J., Hauser, M., Gallinat, J., Heinz, A., & Haggard, P. (2010). Altered awareness of action in schizophrenia: A specific deficit in predicting action consequences. Brain, 133(10), 3104–3112. doi: https://doi.org/10.1093/brain/awq152
Wegner, D. M. (2003). The mind’s best trick: How we experience conscious will. Trends in Cognitive Sciences, 7(2), 65–69. doi: https://doi.org/10.1016/S1364-6613(03)00002-0
Wegner, D. M., Sparrow, B., & Winerman, L. (2004). Vicarious agency: Experiencing control over the movements of others. Journal of Personality and Social Psychology, 86(6), 838–848. doi: https://doi.org/10.1037/0022-3514.86.6.838
Wegner, D. M., & Wheatley, T. (1999). Apparent mental causation: Sources of the experience of will. American Psychologist, 54(7), 480–492. doi: https://doi.org/10.1037/0003-066X.54.7.480
Weiss, C., Tsakiris, M., Haggard, P., & Schütz-Bosbach, S. (2014). Agency in the sensorimotor system and its relation to explicit action awareness. Neuropsychologia, 52, 82–92. doi: https://doi.org/10.1016/j.neuropsychologia.2013.09.034
Wen, W., Yamashita, A., & Asama, H. (2015). The sense of agency during continuous action: Performance is more important than action-feedback association. PLoS ONE, 10(4), e0125226. doi: https://doi.org/10.1371/journal.pone.0125226
Wenke, D., Fleming, S. M., & Haggard, P. (2010). Subliminal priming of actions influences sense of control over effects of action. Cognition, 115(1), 26–38. doi: https://doi.org/10.1016/j.cognition.2009.10.016
Westfall, J. (2016). PANGEA: Power ANalysis for GEneral Anova designs. Unpublished manuscript. Retrieved from http://jakewestfall.org/publications/pangea.pdf.
Wolpe, N., Haggard, P., Siebner, H. R., & Rowe, J. B. (2013). Cue integration and the perception of action in intentional binding. Experimental Brain Research, 229(3), 467–474. doi: https://doi.org/10.1007/s00221-013-3419-2
Wolpert, D. M., & Flanagan, J. R. (2001). Motor prediction. Current Biology, 11(18), R729–R732. doi: https://doi.org/10.1016/S0960-9822(01)00432-8
Wolpert, D. M., Ghahramani, Z., & Jordan, M. I. (1995). An internal model for sensorimotor integration. Science, 269(5232), 1880–1882.
Wolpert, D. M., & Kawato, M. (1998). Multiple paired forward and inverse models for motor control. Neural Networks, 11(7–8), 1317–1329. doi: https://doi.org/10.1016/S0893-6080(98)00066-5
Wolpert, D. M., Miall, R. C., & Kawato, M. (1998). Internal models in the cerebellum. Trends in Cognitive Sciences, 2(9), 338–347. doi: https://doi.org/10.1016/S1364-6613(98)01221-2
Open practices statement
The data for the experiments reported here are available at https://osf.io/x6qbh/. None of the experiments were preregistered.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyawaki, Y., Morioka, S. Confusion within feedback control between cognitive and sensorimotor agency cues in self-other attribution. Atten Percept Psychophys 82, 3957–3972 (2020). https://doi.org/10.3758/s13414-020-02129-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-020-02129-5