Abstract
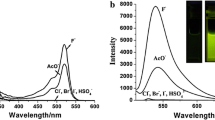
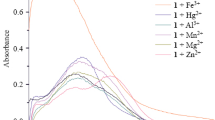
A novel ratiometric fluorescent fluoride probe based on isoquinolinium salts has been designed and synthesized. According to the qualitative and quantitative studies the chemosensor has high selectivity and sensitivity to fluoride. Fluoride induces deprotection of the silyl protecting group of the hydroxyl group and causes a significant change in emission wavelength. The relative fluorescent intensity (I435/I540) increases linearly with F– concentration in the range of 0–10.0 μmol/L. The dynamic determination process is monitored by 1H NMR spectroscopy.






Similar content being viewed by others
REFERENCES
Jagtap, S., Yenkie, M.K., Labhsetwar, N., and Rayalu, S., Chem. Rev., 2012, vol. 112, p. 2454. https://doi.org/10.1021/cr2002855
Zhou, Y., Zhang, J.F., and Yoon, J., Chem. Rev., 2014, vol. 114, p. 5511. https://doi.org/10.1021/cr400352m
Li, Y., Duan, Y., Zheng, J., Li, J., Zhao, W., Yang, S., and Yang, R., Anal. Chem., 2013, vol. 85, p. 11456. https://doi.org/10.1021/ja908215t
Refsnes, M., Schwarze, P.E., Holme, J.A., and Lag, M., Hum. Exp. Toxicol., 2003, vol. 22, p. 111. https://doi.org/10.1191/0960327103ht322oa
Fawell, J., Bailey, K., Chilton, J., Dahi, E., and Magara, Y., WHO Drinking-Water Quality Series, Fluoride in Drinking-Water, London: IWA Publishing, 2006.
Tang, Y., Yu, Y., Wei, X., Yang, J., Zhu, Y., Zhao, Y.H., Tang, Z., Zhou, Z., Li, X., and Yu, X., Tetrahedron Lett., 2019, vol. 60. https://doi.org/10.1016/j.tetlet.2019.151187
Xu, J., Zhang, Y., Yu, H., Gao, X., and Shao, S., Anal. Chem., 2016, vol. 88, p. 1455. https://doi.org/10.1021/acs.analchem.5b04424
Ren, T., Xu, W., Zhang, Q., Zhang, X., Wen, S., Yi, H., Yuan, L., and Zhang. X., Angew. Chem. Int. Ed., 2018, vol. 57, p. 7473. https://doi.org/10.1002/anie.201800293
Zhao, Y.H., Luo, Y., Wang, H., Wei, H., Guo, T., Tan, H., Yuan, L., and Zhang, X.-B., Anal. Chim. Acta, 2019, vol. 1065, p. 134. https://doi.org/10.1016/j.aca.2019.03.029
Gabrielli, L., and Mancin, F., J. Org. Chem., 2016, vol. 81, p. 10715. https://doi.org/10.1021/acs.joc.6b01787
Hisamatsu, S., Suzuki, S., Kohmoto, S., Kishikawa, K., Yamamoto, Y., Motokawa, R., and Yaita, T., Tetrahedron, 2017, vol. 73, p. 3993. https://doi.org/10.1016/j.tet.2017.05.084
Turan, S. and Akkaya, E.U., Org. Lett., 2014, vol. 16, p. 1680. https://doi.org/10.1021/ol5003412
Shi, X., Fan, W., Fan, C., Lu, Z., Bo, Q., Wang, Z., Black, C.A., Wang, F., and Wang, Y., Dyes Pigments, 2017, vol. 140, p. 109. https://doi.org/10.1016/j.dyepig.2017.01.038
Zhao, Y.H., Li, Y., Long, Y., Zhou, Z., Tang, Z., Deng, K., and Zhang, S., Tetrahedron Lett., 2017, vol. 58, p. 1351. https://doi.org/10.1016/j.tetlet.2017.02.066
Wu, Z. and Tang, X., Anal. Chem., 2015, vol. 87, p. 8613. https://doi.org/10.1021/acs.analchem.5b02578
Li, W., Gong, X., Fan, X., Yin, S., Su, D., Zhang, X., and Yuan, L., Chin. Chem. Lett., 2019, vol. 30, p. 1775. https://doi.org/10.1016/j.cclet.2019.07.056
Ye, Z.S., Guo, R.N., Cai, X.F., Chen, M.W., Shi, L., and Zhou, Y.G., Angew. Chem. Int. Ed., 2013, vol. 52, p. 3685. https://doi.org/10.1002/anie.201208300
Zhao, Y.H., Yu, Y., Hu, D., Zhao, L., Xie, W., and Zhou, Z., Asian J. Org. Chem., 2020, vol. 9, p. 953. https://doi.org/10.1002/ajoc.202000202
Funding
The generous financial support from the National Natural Science Foundation of China (nos. 51773057 and 21877034), the Hunan Provincial Education Department Scientific Research Fund (no. 18B221) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Zhu, Y., Yu, Y., Zhao, YH. et al. Design, Synthesis, and Applications of a Novel Fluoride Probe Based on Isoquinolinium Salt. Russ J Gen Chem 90, 1518–1522 (2020). https://doi.org/10.1134/S1070363220080204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220080204