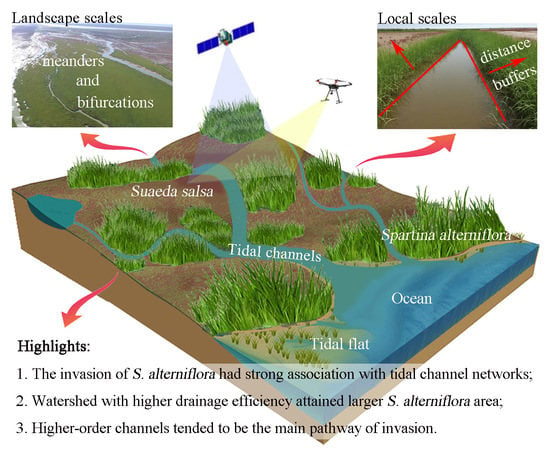
How Does Spartina alterniflora Invade in Salt Marsh in Relation to Tidal Channel Networks? Patterns and Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Acquisition and Preprocessing
2.3. Classification Methods and Accuracy Assessment
2.4. Quantifying Tidal Channel Properties
2.5. Quantifying the Invasion of S. alterniflora
3. Results
3.1. Spatiotemporal Dynamics of S. alterniflora Invasion and Tidal Channel Characteristics
3.2. Relationship between Tidal Channels Networks and S. alterniflora Distribution at Landscape Scales
3.3. Relationship between Tidal Channel Networks and S. alterniflora Invasion at Local Scales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of spartina. Ann. Rev. Ecol. Evol. Syst. 2013, 44, 389–410. [Google Scholar] [CrossRef]
- Zheng, S.; Shao, D.; Sun, T. Productivity of invasive saltmarsh plant Spartina alterniflora along the coast of China: A meta-analysis. Ecol. Eng. 2018, 117, 104–110. [Google Scholar] [CrossRef]
- Ning, Z.; Xie, T.; Liu, Z.; Bai, J.; Cui, B. Native herbivores enhance the resistance of an anthropogenically disturbed salt marsh to Spartina alterniflora invasion. Ecosphere 2019, 10, e02565. [Google Scholar] [CrossRef] [Green Version]
- Daehler, C.C.; Strong, D.R. Status, prediction and prevention of introduced cordgrass Spartina spp. invasions in Pacific estuaries, USA. Biol. Conserv. 1996, 78, 51–58. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Zhu, Z. The range expansion patterns of Spartina alterniflora on salt marshes in the Yangtze Estuary, China. Estuar. Coast. Shelf Sci. 2010, 88, 99–104. [Google Scholar] [CrossRef]
- Davis, H.G.; Taylor, C.M.; Civille, J.C.; Strong, D.R. An Allee effect at the front of a plant invasion: Spartina in a Pacific estuary. J. Ecol. 2004, 321–327. [Google Scholar] [CrossRef]
- Tyler, A.C.; Lambrinos, J.G.; Grosholz, E.D. Nitrogen inputs promote the spread of an invasive marsh grass. Ecol. Appl. 2007, 17, 1886–1898. [Google Scholar] [CrossRef]
- Grosholz, E. Avoidance by grazers facilitates spread of an invasive hybrid plant. Ecol. Lett. 2010, 13, 145–153. [Google Scholar] [CrossRef]
- Marani, M.; Silverstri, S.; Belluco, E.; Camuffo, M.; D’Alpaos, A.; Lanzoni, S.; Marani, A.; Rinaldo, A. Patterns in tidal environments: Salt-marsh channel networks and vegetation. in IGARSS. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IEEE Cat. No. 03CH37477), Toulouse, France, 21–25 July 2003. [Google Scholar]
- Kim, D.; Cairns, D.M.; Bartholdy, J. Tidal creek morphology and sediment type influence spatial trends in salt marsh vegetation. Prof. Geogr. 2013, 65, 544–560. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, L.; Zhang, Y.; Weng, Q.; Morris, J. Tidal and meteorological influences on the growth of invasive spartina alterniflora: Evidence from UAV remote sensing. Remote Sens. 2019, 11, 1208. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yu, Q.; Wang, Y.; Wang, Z.B.; Gao, S.; Flemming, B. The relationship between inundation duration and Spartina alterniflora growth along the Jiangsu coast, China. Estuar. Coast. Shelf Sci. 2018, 213, 305–313. [Google Scholar] [CrossRef]
- Schwarz, C.; Ysebaert, T.; Vandenbruwaene, W.; Temmerman, S.; Zhang, L.; Herman, P.M.J. On the potential of plant species invasion influencing bio-geomorphologic landscape formation in salt marshes. Earth Surf. Process. Landf. 2016, 41, 2047–2057. [Google Scholar] [CrossRef]
- Tyler, A.C.; Zieman, J.C. Patterns of development in the creekbank region of a barrier island Spartina alterniflora marsh. Mar. Ecol. Prog. Ser. 1999, 180, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Shao, D.; Gualtieri, C.; Purnama, A.; Cui, B. Modelling long-distance floating seed dispersal in salt marsh tidal channels. Ecohydrology 2020, 13, e2157. [Google Scholar] [CrossRef]
- Shimamura, R.; Kachi, N.; Kudoh, H.; Whigham, D.F. Hydrochory as a determinant of genetic distribution of seeds within Hibiscus moscheutos (Malvaceae) populations. Am. J. Bot. 2007, 94, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Van der Stocken, T.; Vanschoenwinkel, B.; de Ryck, D.J.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. Interaction between water and wind as a driver of passive dispersal in mangroves. PLoS ONE 2015, 10, e0121593. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Sun, T.; Zhang, H.; Zhu, M.; Yang, W.; Shao, D.; Voinov, A. Maintenance of salt barrens inhibited landward invasion of Spartina species in salt marshes. Ecosphere 2017, 8, e01982. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Li, Y.; Liu, L.; Xie, F. Recognition of spatial expansion patterns of invasive Spartina alterniflora and simulation of the resulting landscape-changes. Acta Ecol. Sin. 2018, 38, 5413–5422. (In Chinese) [Google Scholar]
- Sanderson, E.W.; Zhang, M.; Ustin, S.L.; Rejmankova, E. Geostatistical scaling of canopy water content in a California salt marsh. Landsc. Ecol. 1998, 13, 79–92. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, Y.; Tian, B.; Ding, X. The spatial relationship between salt marsh vegetation patterns, soil elevation and tidal channels using remote sensing at Chongming Dongtan Nature Reserve, China. Acta Oceanol. Sin. 2016, 35, 26–34. [Google Scholar] [CrossRef]
- Lathrop, R.G.; Windham, L.; Montesano, P. Does phragmites expansion alter the structure and function of marsh landscapes? Patterns and processes revisited. Estuaries 2003, 26, 435. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Ustin, S.L.; Foin, T.C. The influence of tidal channels on the distribution of salt marsh plant species in Petaluma Marsh, CA, USA. Plant Ecol. 2000, 146, 29–41. [Google Scholar] [CrossRef]
- Chapple, D.; Dronova, I. Vegetation development in a tidal marsh restoration project during a historic drought: A remote sensing approach. Front. Mar. Sci. 2017, 4, 243. [Google Scholar] [CrossRef] [Green Version]
- Brand, L.A.; Smith, L.M.; Takekawa, J.Y.; Athearn, N.D.; Taylor, K.; Shellenbarger, G.G.; Schoellhamer, D.H.; Spenst, R. Trajectory of early tidal marsh restoration: Elevation, sedimentation and colonization of breached salt ponds in the northern San Francisco bay. Ecol. Eng. 2012, 42, 19–29. [Google Scholar] [CrossRef]
- Moffett, K.B.; Robinson, D.A.; Gorelick, S.M. Relationship of salt marsh vegetation zonation to spatial patterns in soil moisture, salinity, and topography. Ecosystems 2010, 13, 1287–1302. [Google Scholar] [CrossRef] [Green Version]
- Kearney, W.S.; Fagherazzi, S. Salt marsh vegetation promotes efficient tidal channel networks. Nat. Commun. 2016, 7, 12287. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, E.W.; Foin, T.C.; Ustin, S.L. A simple empirical model of salt marsh plant spatial distributions with respect to a tidal channel network. Ecol. Modell. 2001, 139, 293–307. [Google Scholar] [CrossRef]
- Hou, M.; Liu, H.; Zhang, H. Effection of tidal creek system on the expansion of the invasive Spartina in the coastal wetland of Yancheng. Acta Ecol. Sin. 2014, 34, 400–409. (In Chinese) [Google Scholar]
- Chirol, C.; Haigh, I.D.; Pontee, N.; Thompson, C.E.; Gallop, S.L. Parametrizing tidal creek morphology in mature saltmarshes using semi automated extraction from lidar. Remote Sens. Environ. 2018, 209, 291–311. [Google Scholar] [CrossRef]
- Marani, M.; Belluco, E.; D’Alpaos, A.; Defina, A.; Lanzoni, S.; Rinaldo, A. On the drainage density of tidal networks. Water Resour. Res. 2003, 39. [Google Scholar] [CrossRef]
- Maheu-Giroux, M.; de Blois, S. Landscape ecology of phragmites australis invasion in networks of linear wetlands. Landsc. Ecol. 2006, 22, 285–301. [Google Scholar] [CrossRef]
- Xie, T.; Cui, B.; Li, S.; Zhang, S. Management of soil thresholds for seedling emergence to re-establish plant species on bare flats in coastal salt marshes. Hydrobiologia 2019, 827, 51–63. [Google Scholar] [CrossRef]
- Kong, D.; Miao, C.; Borthwick, A.G.L.; Duan, Q.; Liu, H.; Sun, Q.; Ye, A.; Di, Z.; Gong, W. Evolution of the Yellow River Delta and its relationship with runoff and sediment load from 1983 to 2011. J. Hydrol. 2015, 520, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Bai, J.; Jia, J.; Wang, X.; Wang, W.; Zhao, Q.; Zhang, S. Soil organic carbon contents and stocks in coastal salt marshes with spartina alterniflora following an invasion chronosequence in the Yellow River Delta, China. Chin. Geograph. Sci. 2018, 28, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Cui, B.; Xie, T.; Zhang, K. Diversity pattern of macrobenthos associated with different stages of wetland restoration in the Yellow River Delta. Wetlands 2016, 36, 57–67. [Google Scholar] [CrossRef]
- Mao, D.; Liu, M.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Zhang, Y. Rapid invasion of spartina alterniflora in the coastal zone of Mainland China: Spatiotemporal patterns and human prevention. Sensors 2019, 19, 2308. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Mao, D.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Ren, C.; Zhang, Y. Rapid invasion of spartina alterniflora in the coastal zone of Mainland China: New observations from landsat OLI images. Remote Sens. 2018, 10, 1933. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Cui, B.; Luo, M. Effectiveness of microtopographic structure in species recovery in degraded salt marshes. Mar. Pollut. Bull. 2018, 133, 173–181. [Google Scholar] [CrossRef]
- Liu, W.; Maung-Douglass, K.; Strong, D.R.; Pennings, S.C.; Zhang, Y.; Mack, R. Geographical variation in vegetative growth and sexual reproduction of the invasive Spartina alterniflora in China. J. Ecol. 2016, 104, 173–181. [Google Scholar] [CrossRef]
- Fan, H.; Huang, H. Spatial-temporal changes of tidal flats in the Huanghe River Delta using Landsat TM/ETM+ images. J. Geograph. Sci. 2004, 14, 366–374. [Google Scholar]
- Vandenbruwaene, W.; Bouma, T.J.; Meire, P.; Temmerman, S. Bio-geomorphic effects on tidal channel evolution: Impact of vegetation establishment and tidal prism change. Earth Surf. Process. Landf. 2013, 38, 122–132. [Google Scholar] [CrossRef]
- Strahler, A.N. Part II. Quantitative geomorphology of drainage basins and channel networks. In Handbook of Applied Hydrology; McGraw-Hill: New York, NY, USA, 1964; pp. 4–39. [Google Scholar]
- Ren, G.; Liu, Y.; Ma, Y.; Zhang, J. Spartina alterniflora monitoring and change analysis in yellow river delta by remote sensing technology. Acta Laser Biol. Sin. 2014, 23, 515–523. [Google Scholar]
- Ai, J.; Gao, W.; Gao, Z.; Shi, R.; Zhang, C. Phenology-based Spartina alterniflora mapping in coastal wetland of the Yangtze Estuary using time series of GaoFen satellite no. 1 wide field of view imagery. J. Appl. Remote Sens. 2017, 11, 026020. [Google Scholar] [CrossRef]
- Ma, X.; Qiu, D.; Wang, F.; Jiang, X.; Sui, H.; Liu, Z.; Yu, S.; Ning, Z.; Gao, F.; Bai, J.; et al. Tolerance between non-resource stress and an invader determines competition intensity and importance in an invaded estuary. Sci. Total Environ. 2020, 724, 138225. [Google Scholar] [CrossRef]
- Ren, G.B.; Wang, J.J.; Wang, A.D.; Wang, J.B.; Zhu, Y.L.; Wu, P.Q.; Ma, Y.; Zhang, J. Monitoring the invasion of smooth cordgrass spartina alterniflora within the modern yellow river delta using remote sensing. J. Coast. Res. 2019, 90, 135–145. [Google Scholar] [CrossRef]
- Hou, C.; Song, J.; Yan, J.; Wang, K.; Li, C.; Yi, Y. Growth indicator response of Zostera japonica under different salinity and turbidity stresses in the Yellow River Estuary, China. Mar. Geol. 2020, 424, 106169. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Li, L.; Man, W.; Jia, M.; Wang, Z.; Lu, C. Monitoring the Invasion of Spartina alterniflora Using Multi-source High-resolution Imagery in the Zhangjiang Estuary, China. Remote Sens. 2017, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices; CRC Press: Boka Raton, FL, USA, 2019. [Google Scholar]
- Lohani, B.; Mason, D.; Scott, T.R.; Sreenivas, B. Extraction of tidal channel networks from aerial photographs alone and combined with laser altimetry. Int. J. Remote Sens. 2006, 27, 5–25. [Google Scholar] [CrossRef]
- D’Alpaos, A.; Lanzoni, S.; Marani, M.; Bonornetto, A.; Cecconi, G.; Rinaldo, A. Spontaneous tidal network formation within a constructed salt marsh: Observations and morphodynamic modelling. Geomorphology 2007, 91, 186–197. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.; Ene, E. Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst. 2012. Available online: umassedu/landeco/research/fragstats/fragstatshtml (accessed on 23 January 2015).
- An, S.Q.; Gu, B.H.; Zhou, C.F.; Wang, Z.S.; Deng, Z.F.; Zhi, Y.B.; Li, H.L.; Chen, L.; Yu, D.H.; Liu, Y.H. Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 2007, 47, 183–191. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Furbish, D.J. On the shape and widening of salt marsh creeks. J. Geophys. Res. Oceans 2001, 106, 991–1003. [Google Scholar] [CrossRef]
- Allen, J.R.L. Morphodynamics of holocene salt marshes: A review sketch from the Atlantic and Southern North Sea coasts of Europe. Quat. Sci. Rev. 2000, 19, 1155–1231. [Google Scholar] [CrossRef]
- Reed, D.J.; Spencer, T.; Murray, A.L.; French, J.R.; Leonard, L. Marsh surface sediment deposition and the role of tidal creeks: Implications for created and managed coastal marshes. J. Coast. Conserv. 1999, 5, 81–90. [Google Scholar] [CrossRef]
- Kim, D.; Cairns, D.M.; Bartholdy, J. Environmental controls on multiscale spatial patterns of salt marsh vegetation. Phys. Geograph. 2013, 31, 58–78. [Google Scholar] [CrossRef]
- Howes, B.L.; Goehringer, D.D. Porewater drainage and dissolved organic carbon and nutrient losses through the intertidal creekbanks of a New England salt marsh. Mar. Ecol. Progress Ser. Oldend. 1994, 114, 289–301. [Google Scholar] [CrossRef]
- Le Hir, P.; Roberts, W.; Cazaillet, O.; Christie, M.; Bassoullet, P.; Bacher, C. Characterization of intertidal flat hydrodynamics. Continent. Shelf Res. 2000, 20, 1433–1459. [Google Scholar] [CrossRef] [Green Version]
- Ayres, D.R.; Smith, D.L.; Zaremba, K.; Klohr, S.; Strong, D.R. Spread of exotic cordgrasses and hybrids (Spartina sp.) in the tidal marshes of San Francisco Bay, California, USA. Biol. Invasions 2004, 6, 221–231. [Google Scholar] [CrossRef]









| Year | Source | Acquisition Date | Resolution (m) of Multispectral Bands | Resolution (m) of Pansharpen Bands | Tides |
|---|---|---|---|---|---|
| 2010 | Landsat 7 ETM+ | 5 October 2010 | 30 | 15 | Low |
| 2011 | Landsat 7 ETM+ | 11 December 2011 | 30 | 15 | Low |
| 2012 | Landsat 7 ETM+ | 27 November 2012 | 30 | 15 | Low |
| 2013 | Landsat 8 OLI | 22 November 2013 | 30 | 15 | Low |
| 2014 | Landsat 8 OLI | 24 October 2014 | 30 | 15 | Low |
| 2015 | Landsat 8 OLI | 27 October 2015 | 30 | 15 | Low |
| 2016 | Landsat 8 OLI | 16 December 2016 | 30 | 15 | Low |
| 2017 | Landsat 8 OLI | 30 September 2017 | 30 | 15 | Low |
| 2018 | Landsat 8 OLI | 3 October 2018 | 30 | 15 | Low |
| 2014 | GaoFen -1 PMS2 | 21 May 2014 | 8 | 2 | Middle |
| 2015 | GaoFen -1 PMS2 | 25 May 2015 | 8 | 2 | Low |
| 2016 | GaoFen -2 PMS2 | 11 August 2016 | 4 | 1 | High |
| 2017 | GaoFen -2 PMS2 | 31 January 2017 | 4 | 1 | Low |
| 2018 | GaoFen -2 PMS2 | 1 March 2018 | 4 | 1 | High |
| Watershed | Total Channel Length (km) | Watershed Area (km2) | Higher-Order Channel Length (km) | Maximum Channel Mouth Width (m) | OPL (m) |
|---|---|---|---|---|---|
| #1 | 39.196 | 8.417 | 17.603 | 98.613 | 163.934 |
| #2 | 27.296 | 4.280 | 8.641 | 81.759 | 181.818 |
| #3 | 19.580 | 3.065 | 8.096 | 50.408 | 188.679 |
| #4 | 48.281 | 7.078 | 10.332 | 132.016 | 140.845 |
| #5 | 42.227 | 7.388 | 12.556 | 102.916 | 169.492 |
| #6 | 67.572 | 15.716 | 19.955 | 134.704 | 200.000 |
| Total | 244.153 | 45.943 | 77.758 | — | — |
| Year | Annual Invasion Rate (p) of S. alterniflora | ||||||
|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | Total | |
| 2010–2011 | 4.45 | — | — | 14.50 | — | — | 4.47 |
| 2011–2012 | 0.41 | — | — | 22.48 | — | — | 0.66 |
| 2012–2013 | 0.36 | 16.49 | — | 7.28 | 13.49 | — | 1.95 |
| 2013–2014 | 0.08 | 0.07 | — | 0.17 | 1.01 | 4.48 | 0.23 |
| 2014–2015 | 0.96 | 0.16 | 45.83 | 0.63 | 0.59 | 0.08 | 0.71 |
| 2015–2016 | 0.27 | 0.25 | 0.48 | 0.41 | 0.4 | 2.07 | 0.41 |
| 2016–2017 | 0.17 | 0.06 | 0.05 | 0.02 | 0.04 | 0.06 | 0.10 |
| 2017–2018 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.16 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Shao, D.; Xie, T.; Gao, W.; Ma, X.; Ning, Z.; Cui, B. How Does Spartina alterniflora Invade in Salt Marsh in Relation to Tidal Channel Networks? Patterns and Processes. Remote Sens. 2020, 12, 2983. https://doi.org/10.3390/rs12182983
Sun L, Shao D, Xie T, Gao W, Ma X, Ning Z, Cui B. How Does Spartina alterniflora Invade in Salt Marsh in Relation to Tidal Channel Networks? Patterns and Processes. Remote Sensing. 2020; 12(18):2983. https://doi.org/10.3390/rs12182983
Chicago/Turabian StyleSun, Limin, Dongdong Shao, Tian Xie, Weilun Gao, Xu Ma, Zhonghua Ning, and Baoshan Cui. 2020. "How Does Spartina alterniflora Invade in Salt Marsh in Relation to Tidal Channel Networks? Patterns and Processes" Remote Sensing 12, no. 18: 2983. https://doi.org/10.3390/rs12182983