Abstract
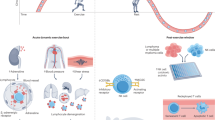
Unhealthful lifestyle factors, such as obesity, disrupt organismal homeostasis and accelerate cancer pathogenesis, partly through metabolic and immunological dysregulation. Exercise is a prototypical strategy that maintains and restores homeostasis at the organismal, tissue, cellular and molecular levels and can prevent or inhibit numerous disease conditions, including cancer. Here, we review unhealthful lifestyle factors that contribute to metabolic and immunological dysregulation and drive tumourigenesis, focusing on patient physiology (host)–tissue–tumour microenvironment interactions. We also discuss how exercise may influence distant tissue microenvironments, thereby improving tissue function through both metabolic and immunospecific pathways. Finally, we consider future directions that merit consideration in basic and clinical translational exercise studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Song, M. & Giovannucci, E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2, 1154–1161 (2016).
Song, M., Vogelstein, B., Giovannucci, E. L., Willett, W. C. & Tomasetti, C. Cancer prevention: molecular and epidemiologic consensus. Science 361, 1317–1318 (2018).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Chovatiya, R. & Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 (2014).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Schwörer, S., Vardhana, S. A. & Thompson, C. B. Cancer metabolism drives a stromal regenerative response. Cell Metab. 29, 576–591 (2019).
Palucka, A. K. & Coussens, L. M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Wang, A., Luan, H. H. & Medzhitov, R. An evolutionary perspective on immunometabolism. Science 363, eaar3932 (2019).
Mehla, K. & Singh, P. K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer 5, 822–834 (2019).
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G. & Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 30, 36–50 (2019).
Dyck, L. & Lynch, L. Cancer, obesity and immunometabolism: connecting the dots. Cancer Lett. 417, 11–20 (2018).
Turbitt, W. J., Buchta Rosean, C., Weber, K. S. & Norian, L. A. Obesity and CD8 T cell metabolism: implications for anti-tumor immunity and cancer immunotherapy outcomes. Immunol. Rev. 295, 203–219 (2020).
Drew, D. A., Cao, Y. & Chan, A. T. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat. Rev. Cancer 16, 173–186 (2016).
Demierre, M. F., Higgins, P. D., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nat. Rev. Cancer 5, 930–942 (2005).
Zaleska, M., Mozenska, O. & Bil, J. Statins use and cancer: an update. Future Oncol. 14, 1497–1509 (2018).
Barron, T. I., Connolly, R. M., Sharp, L., Bennett, K. & Visvanathan, K. Beta blockers and breast cancer mortality: a population-based study. J. Clin. Oncol. 29, 2635–2644 (2011).
Ganz, P. A., Habel, L. A., Weltzien, E. K., Caan, B. J. & Cole, S. W. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res. Treat. 129, 549–556 (2011).
Pernicova, I. & Korbonits, M. Metformin: mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 10, 143–156 (2014).
Moore, S. C. et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 176, 816–825 (2016). Meta-analysis showing that high versus low levels of leisure-time physical activity are associated with lower risk of 13 cancers.
Friedenreich, C. M., Neilson, H. K., Farris, M. S. & Courneya, K. S. Physical activity and cancer outcomes: a precision medicine approach. Clin. Cancer Res. 22, 4766–4775 (2016). Systematic review showing that postdiagnosis physical activity is associated with lower risks of cancer recurrence or progression across breast, prostate and colorectal cancers.
Ashcraft, K. A., Peace, R. M., Betof, A. S., Dewhirst, M. W. & Jones, L. W. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 76, 4032–4050 (2016).
Koelwyn, G. J., Quail, D. F., Zhang, X., White, R. M. & Jones, L. W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 17, 620–632 (2017).
Golemis, E. A. et al. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 32, 868–902 (2018).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Hopkins, B. D., Goncalves, M. D. & Cantley, L. C. Obesity and cancer mechanisms: cancer metabolism. J. Clin. Oncol. 34, 4277–4283 (2016).
Font-Burgada, J., Sun, B. & Karin, M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62 (2016).
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276 (2016).
Howe, L. R., Subbaramaiah, K., Hudis, C. A. & Dannenberg, A. J. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 19, 6074–6083 (2013).
Clements, V. K. et al. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J. Leukoc. Biol. 103, 395–407 (2018).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
Baek, A. E. et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 8, 864 (2017).
Zhang, C. et al. STAT3 activation-induced fatty acid oxidation in CD8+ T effector cells is critical for obesity-promoted breast tumour growth. Cell Metab. 31, 148–161.e145 (2020).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Krall, J. A. et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 10, eaan3464 (2018).
Koelwyn, G.J. et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med. https://doi.org/10.1038/s41591-020-0964-7 (2020).
Meijers, W. C. et al. Heart failure stimulates tumor growth by circulating factors. Circulation 138, 678–691 (2018).
Hasin, T. et al. Heart failure after myocardial infarction is associated with increased risk of cancer. J. Am. Coll. Cardiol. 68, 265–271 (2016).
Hasin, T. et al. Patients with heart failure have an increased risk of incident cancer. J. Am. Coll. Cardiol. 62, 881–886 (2013).
Peake, J. M. et al. Modulating exercise-induced hormesis: does less equal more? J. Appl. Physiol. 119, 172–189 (2015).
Hawley, J. A., Hargreaves, M., Joyner, M. J. & Zierath, J. R. Integrative biology of exercise. Cell 159, 738–749 (2014).
Egan, B., Hawley, J. A. & Zierath, J. R. SnapShot: exercise metabolism. Cell Metab. 24, 342–342.e341 (2016).
Koelwyn, G. J., Wennerberg, E., Demaria, S. & Jones, L. W. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncol. (Williston Park) 29, 908–920 (2015). 922.
Murphy, R. M., Watt, M. J. & Febbraio, M. A. Metabolic communication during exercise. Nat. Metab. https://doi.org/10.1038/s42255-020-0258-x (2020).
Joyner, M. J. & Dempsey, J. A. Physiological redundancy and the integrative responses to exercise. Cold Spring Harb. Perspect. Med. 8, a029660 (2018).
Jones, L. W., Eves, N. D., Haykowsky, M., Freedland, S. J. & Mackey, J. R. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 10, 598–605 (2009).
Neufer, P. D. et al. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 22, 4–11 (2015).
Severinsen, M. C. K. & Pedersen, B. K. Muscle-organ crosstalk: the emerging roles of myokines. Endocr. Rev. 41, 594–609 (2020).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Michelotti, G. A., Machado, M. V. & Diehl, A. M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10, 656–665 (2013).
Gehrke, N. et al. Voluntary exercise in mice fed an obesogenic diet alters the hepatic immune phenotype and improves metabolic parameters: an animal model of life style intervention in NAFLD. Sci. Rep. 9, 4007 (2019). Preclinical study showing that exercise protects against diet-induced NAFLD in mice and improves liver-specific metabolic and inflammatory alterations.
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Olén, O. et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 395, 123–131 (2020).
Qin, L. et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget 8, 7391–7404 (2017). Preclinical study showing exercise protection from chronic colitis alongside decreases in colon-specific inflammation.
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019). Study showing that exercise in mice decreases bone marrow haematopoiesis via leptin signalling and epigenetic alterations in bone marrow hematopoietic progenitors.
Wu, W. C. et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl Acad. Sci. USA 111, 4221–4226 (2014).
Shipp, C., Speigl, L., Janssen, N., Martens, A. & Pawelec, G. A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell. Mol. Life Sci. 73, 4043–4061 (2016).
Stanford, K. I. & Goodyear, L. J. Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 38, 308–314 (2014).
Bigley, A. B. & Simpson, R. J. NK cells and exercise: implications for cancer immunotherapy and survivorship. Discov. Med. 19, 433–445 (2015).
Timmerman, K. L., Flynn, M. G., Coen, P. M., Markofski, M. M. & Pence, B. D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 84, 1271–1278 (2008).
Peters, C., Lötzerich, H., Niemeir, B., Schüle, K. & Uhlenbruck, G. Exercise, cancer and the immune response of monocytes. Anticancer Res. 15, 175–179 (1995).
Fairey, A. S. et al. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J. Appl. Physiol. 98, 1534–1540 (2005).
Irwin, M. L. et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol. Biomark. Prev. 18, 306–313 (2009).
Fairey, A. S. et al. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 12, 721–727 (2003).
Zhu, Z. et al. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol. Biomark. Prev. 17, 1920–1929 (2008).
Xie, L. et al. Effects of dietary calorie restriction or exercise on the PI3K and Ras signaling pathways in the skin of mice. J. Biol. Chem. 282, 28025–28035 (2007).
Aveseh, M., Nikooie, R. & Aminaie, M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J. Physiol. (Lond.) 593, 2635–2648 (2015).
Lu, M. et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab. 6, 14 (2018). Study showing differential sensitivity to exercise across PDX models, occurring in conjunction with whole-tumour metabolic alterations.
Glass, O. K. et al. Differential response to exercise in claudin-low breast cancer. Oncotarget 8, 100989–101004 (2017).
Zielinski, M. R., Muenchow, M., Wallig, M. A., Horn, P. L. & Woods, J. A. Exercise delays allogeneic tumour growth and reduces intratumoural inflammation and vascularization. J. Appl. Physiol. 96, 2249–2256 (2004).
Almeida, P. W. et al. Swim training suppresses tumour growth in mice. J. Appl. Physiol. 107, 261–265 (2009).
Pedersen, L. et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 23, 554–562 (2016). Study showing that exercise inhibits tumour growth across multiple preclinical models, including the B16 mouse melanoma model, in a manner dependent on NK-cell tumour infiltration.
Hagar, A. et al. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 19, 536 (2019).
Sio, A. et al. Dysregulated hematopoiesis caused by mammary cancer is associated with epigenetic changes and hox gene expression in hematopoietic cells. Cancer Res. 73, 5892–5904 (2013).
Wennerberg, E. et al. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 11, 452–461 (2020).
Zois, C. E. & Harris, A. L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. (Berl.) 94, 137–154 (2016).
Dauer, P. & Lengyel, E. New roles for glycogen in tumor progression. Trends Cancer 5, 396–399 (2019).
Vande Voorde, J. et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 5, eaau7314 (2019).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e217 (2017).
Day, C. P., Merlino, G. & Van Dyke, T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163, 39–53 (2015).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Betof, A. S. et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J. Natl Cancer Inst. 107, djv040 (2015).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Courneya, K. S. et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr. Oncol. 15, 279–285 (2008).
Newton, R. U. et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open 8, e022899 (2018).
Iyengar, N.M. & Jones, L.W. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2585 (2019).
McTiernan, A. et al. Effect of a 12-month exercise intervention on patterns of cellular proliferation in colonic crypts: a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 15, 1588–1597 (2006).
Campbell, K. L. et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 16, 1767–1774 (2007).
Ligibel, J. A. et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: results from the Pre-Operative Health and Body (PreHAB) Study. Clin. Cancer Res. 25, 5398–5406 (2019). Window-of-opportunity trial showing enrichment in intratumoural immunological and inflammation-associated pathways after short-term exercise in treatment-naive patients with operable breast cancer.
Sims, A. H., Leggate, M. & Campbell, A. Exercise window trial in newly diagnosed breast cancer-letter. Clin. Cancer Res. 25, 7609–7610 (2019).
Hu, B. C., HuBMAP Consortium. The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574, 187–192 (2019).
Shilo, S., Rossman, H. & Segal, E. Axes of a revolution: challenges and promises of big data in healthcare. Nat. Med. 26, 29–38 (2020).
Acknowledgements
G.J.K. is supported by the Louis and Rachel Rudin Foundation. X.Z. and T.T. are supported in part by Josie Robertson, Rita Allen and V Foundation Scholarships, and the Stanley and Fiona Druckenmiller Center for Lung Cancer Research at MSK. A.S. is supported in part by funding from the National Cancer Institute (DP2 CA225212, U54 CA209975), the Josie Robertson Foundation and the Cancer Research Institute. L.W.J. is supported in part by funding from the National Cancer Institute and AKTIV Against Cancer. This work was supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Author information
Authors and Affiliations
Contributions
G.J.K. and L.W.J. researched data for the article, made substantial contributions to the discussion of content and wrote the manuscript. X.Z., T.T. and A.S. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.W.J. owns stock in Pacylex, Inc. G.J.K., X.Z., T.T. and A.S. declare no competing interests.
Additional information
Peer review information Primary Handling Editors: George Caputa; Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koelwyn, G.J., Zhuang, X., Tammela, T. et al. Exercise and immunometabolic regulation in cancer. Nat Metab 2, 849–857 (2020). https://doi.org/10.1038/s42255-020-00277-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00277-4
This article is cited by
-
Efficacy of physical exercise intervention on children with acute lymphoblastic leukemia during treatment and rehabilitation: a systematic review and meta-analysis
Supportive Care in Cancer (2024)
-
Enabling exercise prescription for survivors of cancer
Scientific Reports (2021)
-
Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming
Nature Metabolism (2021)
-
Run for your life: an integrated virtual tissue platform for incorporating exercise oncology into immunotherapy
Cancer Immunology, Immunotherapy (2021)