Abstract
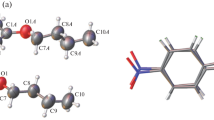
The structure and thermal properties of azobenzene derivatives R1–C6H4–N=N–C6H4–R2, where R1/R2 = CH3COO/C2H5O (I), CH2=C(CH3)COO/C2H5 (II), or C6H13COO/C2H5O (III), were studied by differential scanning calorimetry and X-ray diffraction. Compounds I and III form a mesophase (nematic) upon melting, whereas compound II does not produce a mesophase. Compound III is characterized by two high-temperature crystal–crystal phase transitions IIIIII–IIIII–IIII. The low-temperature crystal modification, which is not a precursor of the mesophase, was structurally characterized. The crystal packing of I is stabilized by C−H···π interactions and consists of alternating loosely and closely packed regions. Weak directional interactions that connect the molecules into zigzag chains ensure the structuring of the mesophase of I. The possible structuring pattern of the mesophase of III is discussed.







Similar content being viewed by others
REFERENCES
O. Lehman, Z. Phys. Chem. 4, 462 (1889).
W. Maier and A. Saupe, Z. Naturforsch. 15a, 287 (1960).
R. L. Humphrie, P. G. Jame, and G. R. Luckhurs, J. Chem. Soc. Faraday Trans. II 68, 1031 (1972).
P. G. de Gennes, Mol. Cryst. Liq. Cryst. 21, 49 (1973).
W. L. Mc Millan, Phys. Rev. A 8, 1921 (1973).
A. Wulf, Phys. Rev. A 11, 365 (1975).
M. A. Cotter, Mol. Cryst. Liq. Cryst. 97, 29 (1983).
M. A. Osipov, Molecular Theories of Liquid Crystals, Sect. 2, Ch. III, Vol. 1: Handbook of Liquid Crystals, Ed. by D. Demus et al. (Wiley–VCH, Weinheim, 1998), p. 40.
G. Vertoge and W. H. Jeu, Thermotropic Liquid Crystals, Fundamentals (Springer, Berlin, 1988).
P. G. de Gennes and J. Prost, The Physics of Liquid Crystals (Oxford Univ. Press, New York, 1995).
S. Singh, Phys. Rep. 324 (2–4), 107 (2000).
L. G. Kuz’mina, I. I. Konstantinov, and S. I. Bezzubov, Khim. Vys. Energ. 50 (6), 478 (2016).
A. N. Kochetov, L. G. Kuz’mina, A. V. Churakov, et al., Crystallogr. Rep. 51 (1), 53 (2006).
L. G. Kuz’mina, N. S. Kucherepa, S. M. Pestov, et al., Crystallogr. Rep. 54 (5), 862 (2009).
L. G. Kuz’mina, S. M. Pestov, A. N. Kochetov, et al., Crystallogr. Rep. 55 (5), 786 (2010).
L. G. Kuz’mina and N. S. Kucherepa, Crystallogr. Rep. 56 (2), 242 (2011).
L. G. Kuz’mina, N. S. Kucherepa, and A. V. Churakov, Crystallogr. Rep. 56 (2), 213 (2012).
L. G. Kuz’mina, M. A. Navasardyan, A. V. Churakov, and J. A. K. Howard, Mol. Cryst. Liq. Cryst. 638, 60 (2016).
I. I. Konstantinov, A. V. Churakov, and L. G. Kuz’mina, Crystallogr. Rep. 58 (1), 81 (2013).
L. G. Kuz’mina, I. I. Konstantinov, and E. Kh. Lermontova, Mol. Cryst. Liq. Cryst. 588, 1 (2014).
L. G. Kuz’mina, I. I. Konstantinov, A. V. Churakov, and M. A. Navasardyan, Acta Crystallogr. E 73, 1052 (2017).
A. I. Vogel, Practical Organic Chemistry, 3rd Ed. (Longman, London, 1966).
Bruker, APEX2, SADABS and SAINT (Bruker AXS Inc., Madison, Wisk, 2008).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
G. R. Desiraju and T. Syeiner, The Weak Hydrogen Bond in Structural Chemistry and Biology (Oxford Univ. Press, Oxford, 1999).
A. Nangia, Cryst. Eng. Commun. 4 (17), 93 (2002).
K. Muller-Dethlefs and P. Hobza, Chem. Rev. 100, 143 (2000).
Ch. Janiak, J. Chem. Soc. Dalton Trans., 3885 (2000).
Funding
This study was supported by the Russian Science Foundation (grant no. 16-13-10273). The samples were synthesized and characterized by I.I. Konstantinov within the framework of the state assignment of the Topchiev Institute of Petrochemical Synthesis of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by T. Safonova
Rights and permissions
About this article
Cite this article
Kuz’mina, L.G., Konstantinov, I.I. & Navasardyan, M.A. Crystal Packing of Potentially Mesomorphic Azobenezene Derivatives R1–C6H4–N=N–C6H4–R2 (R1, R2 = CH3COO, C2H5O; CH2=C(CH3)COO, C2H5; C6H13COO, C2H5O). Crystallogr. Rep. 65, 436–443 (2020). https://doi.org/10.1134/S1063774520030189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774520030189