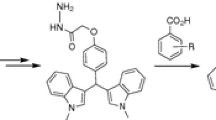
A series of 3,3-bis(indol-3-yl)-1,3-dihydroindol-2-ones containing substituents at different positions of the oxindole ring were synthesized to study the effect of the position of the substituent on biological activity. Some of the new derivatives showed high in vitro cytotoxic activity (MTT assay) on human tumor cell lines and lower (60 and 150 times less) cytotoxicity on donor human fibroblasts compared to doxorubicin.
Similar content being viewed by others
References
Dothager, R. S.; Putt, K. S.; Allen, B. J.; Leslie, B. J.; Nesterenko, V.; Hergenrother, P. J. J. Am. Chem. Soc. 2005, 127, 8686.
Palchaudhuri, R.; Nesterenko, V.; Hergenrother, P. J. J. Am. Chem. Soc. 2008, 130, 10274.
Palchaudhuri, R.; Hergenrother, P. J. Bioorg. Med. Chem. Lett. 2008, 18, 5888.
Al-Qawasmeh, R. A.; Lee, Y.; Cao, M.-Y.; Gu, X.; Vassilakos, A.; Wright, J. A.; Young, A. Bioorg. Med. Chem. Lett. 2004, 14, 347.
Lavrenov, S. N.; Luzikov, Y. N.; Bykov, E. E.; Reznikova, M. I; Stepanova, E. V.; Glazunova, V. A.; Volodina, Y. L.; Tatarsky, V. V., Jr.; Shtil, A. A.; Preobrazhenskaya, M. N. Bioorg. Med. Chem. 2010, 18, 6905.
Stepanova, E. V.; Shtil’, A. A.; Lavrenov, S. N.; Bukhman, V. M.; Inshakov, A. N.; Mirchink, E. P.; Trenin, A. S.; Galatenko, O. A.; Isakova, E. B.; Glazunova, V. A; Dezhenkova, L. G.; Solomko, E. Sh.; Bykov, E. E.; Preobrazhenskaya, M. N. Russ. Chem. Bull., Int. Ed. 2010, 59, 2259. [Izv. Akad. Nauk, Ser. Khim. 2010, 59, 2203.]
Paira, P.; Hazra, A.; Kumar, S.; Paira, R.; Sahu, K. B.; Naskar, S.; Saha, P.; Mondal, S.; Maity, A.; Banerjee, S.; Mondal, N. B. Bioorg. Med. Chem. Lett. 2009, 19, 4786.
Kamal, A.; Srikanth, Y. V. V.; Naseer, M.; Khan, A.; Shaik, T. B.; Ashraf, Md. Bioorg. Med. Chem. Lett. 2010, 20, 5229.
Subba Reddy, B. V.; Rajeswari, N.; Sarangapani, M.; Prashanthi, Y.; Ganji, R. J.; Addlagatta, A. Bioorg. Med. Chem. Lett. 2012, 22, 2460.
Natarajan, A.; Fan, Y.-H.; Chen, H.; Guo, Y.; Iyasere, J.; Harbinski, F.; Christ, W. J.; Aktas, H.; Halperin, J. A. J. Med. Chem. 2004, 47, 1882.
Kleeblatt, D.; Christoph, A.; Cordes, C. A.; Lebrenz, P.; Hein, M.; Feist, H.; Matin, A.; Raza, R.; Iqbal, J.; Munshi, O.; Rahman, Q.; Villinger, A.; Langer, P. RSC Adv. 2014, 4, 22828.
Praveen, C.; Ayyanar, A.; Perumal, P. T. Bioorg. Med. Chem. Lett. 2011, 21, 4072.
Prathima, P. S.; Pamanji Rajesh, P.; Rao, J. V.; Kailash, U. S.; Sridhar, B.; Rao, M. M. Eur. J. Med. Chem. 2014, 84, 155.
Bergman, J.; Eklund, N. Tetrahedron 1980, 36, 1445.
Suresh, B.; Brahmeshwary, G.; Swamy, T.; Gopi, I.; Ravinder, V. Russ. J. Gen. Chem. 2016, 86, 1144.
Gao, G.; Han, Y.; Zhang, Z.-H. ChemistrySelect 2017, 2, 11561.
Liu, X.; Ma, S.; Toy, P. H. Org. Lett. 2019, 21, 9212.
Etayo, P.; Escudero-Adána, E. C.; Pericàs, M. A. Catal. Sci. Technol. 2017, 7, 4830.
(а) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third International Supplement; Clinical and Laboratory Standards Institute: Pensylvania, 2013, CLSI M27-S3. http://medicine.kaums.ac.ir/UploadedFiles/angalshenase/M27-S3%20Third% 20International%20Supplement.pdf. a Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard–Second Edition; Clinical and Laboratory Standards Institute: Pensylvania, 2008, CLSI M38-A2. https://clsi.org/media/1455/m38a2_sample.pdf.
Preobrazhenskaya, M. N.; Olsufyeva, E. N.; Tevyashova, A. N.; Printsevskaya, S. S.; Solovieva, S. E.; Reznikova, M. I.; Trenin, A. S.; Galatenko, O. A.; Treshalin, I. D.; Pereverzeva, E. R.; Mirchink, E. P.; Zotchev, S. B. J. Antibiot. 2010, 63, 55.
Trenin, A. S. Antibiotiki i khimioterapiya 2013, 58(5/6), 3.
Trenin, A. S.; Tsvigun, E. A.; Bychkova, O. P.; Lavrenov, S. N. Antibiotiki i khimioterapiya 2013, 58(9/10), 3.
Sandmeyer, T. Helv. Chim. Acta 1919, 2, 234.
Alimohammadi, K.; Sarrafi, Y.; Tajbakhsh, M. Monatsh. Chem. 2008, 139, 1037.
Yu, J.; Shen, T.; Lin, Y.; Zhou, Y.; Song, Q. Synth. Commun. 2014, 44, 2029.
The study was supported by the Russian Science Foundation (project No. 16-15-10300).
Victor V. Tatarskiy expresses his personal gratitude for the financial support to the Ministry of Science and Higher Education of the Russian Federation in the framework of the competitiveness program of National University of Science and Technology MISiS (grant Z02-2017-2-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
In memory of Professor Maria Nikolaevna Preobrazhenskaya
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(6), 741–746
Rights and permissions
About this article
Cite this article
Lavrenov, S.N., Bychkova, O.P., Dezhenkova, L.G. et al. Synthesis and study of cytotoxic activity of novel 3,3-bis(indol-3-yl)-1,3-dihydroindol-2-ones. Chem Heterocycl Comp 56, 741–746 (2020). https://doi.org/10.1007/s10593-020-02725-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02725-1