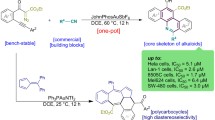
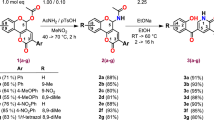
Cage-like amines with norbornane and adamantane frameworks were studied in a versatile, convenient one-pot green synthetic experiment for pyrimidine core annulation via cleavage of a 1H-tetrazole ring. The transannulation was performed without an excess of the reagents and solvent under optimized conditions. As a result, 11 new thieno[2,3-d]pyrimidinones with bulky substituents were obtained in high yields without the need of further purification and with excellent selectivity of the process. The introduced сage-like framework is regarded as a bioisostere of the 2-arylamino moiety. Preliminary screening of the biological activity was performed and 2-{[1-(bicyclo[2.2.1]heptan-2-yl)ethyl]amino}-5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4(3H)-one demonstrated high toxicity toward human leukemia HL-60, cervix carcinoma KB3-1, and colon carcinoma HCT116 cells that correlates well with the results obtained previously for the activity of the compounds with benzylamino substituents.




Similar content being viewed by others
References
Stockdale, T. P.; Williams, C. M. Chem. Soc. Rev. 2015, 44, 7737.
Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd R. Eur. J. Med. Chem. 2011, 46, 1949.
Mykhailiuk, P. K. Org. Biomol. Chem. 2019, 17, 2839.
(a) Tkachenko, I. V.; Tarabara, I. N.; Omelchenko, I. V.; Palchykov, V. A. J. Heterocycl. Chem. 2018, 55, 2381. (b) Kas'yan, L. I.; Prid'ma, S. A.; Turov, A. V.; Pal'chikov, V. A.; Kas'yan, A. O.; Karat, L. D. Russ. J. Org. Chem. 2009, 45, 505. [Zh. Org. Khim. 2009, 45, 520.] (c) Kasyan, L. I.; Sereda, S. V.; Potekhin, K. A.; Kasyan, A. O. Heteroat. Chem. 1997, 8, 177. c Pokhodylo, N. T.; Matiichuk, V. S.; Obushak, M. D. Russ. J. Org. Chem. 2017, 53, 481. [Zh. Org. Khim. 2017, 53, 470.]
Pokhodylo, N. T.; Matiychuk, V. S.; Obushak, M. D. Tetrahedron 2008, 64, 1430.
Pokhodylo, N. T.; Shyyka, O. Ya.; Matiychuk, V. S.; Obushak, M. D. ACS Comb Sci. 2015, 17, 399.
Shyyka, O. Ya.; Pokhodylo, N. T.; Slyvka, Yu. I.; Goreshnik, E. A.; Obushak M. D. Tetrahedron Lett. 2018, 59, 1112.
Obst, M.; König, B. Eur. J. Org. Chem. 2018, 4213.
Molecular Rearrangements in Organic Synthesis; Rojas, C. M., Ed.; Wiley: Hoboken, 2016.
(a) Chattopadhyay, B.; Gevorgyan, V. Angew. Chem., Int. Ed. 2011, 51, 862. (b) Sebris, A.; Turks, M. Chem Heterocycl. Compd. 2019, 55, 1041. [Khim. Geterotsikl. Soedin. 2019, 55, 1041.] (c) Khaidarov, A. R.; Rostovskii, N. V.; Starova, G. L.; Khlebnikov, A. F.; Novikov, M. S. Chem. Heterocycl. Compd. 2018, 54, 946. [Khim. Geterotsikl. Soedin. 2018, 54, 946.]
(a) Shyyka, O. Ya.; Pokhodylo, N. T.; Finiuk, N. S. Biopolym. Cell. 2019, 35, 321. (b) Pokhodylo, N. T.; Shyyka, O. Ya.; Tupychak, M. A.; Obushak, M. D. Chem. Heterocycl. Compd. 2018, 54, 209. [Khim. Geterotsikl. Soedin. 2018, 54, 209.]
Shyyka, O.; Pokhodylo, N.; Finiuk, N.; Matiychuk, V.; Stoika, R.; Obushak, M. Sci. Pharm. 2018, 86(3), 28.
Sokolova, A. S.; Yarovaya, О. I.; Baev, D. S.; Shernyukov, А. V.; Shtro, A. A.; Zarubaev, V. V.; Salakhutdinov, N. F. Eur. J. Med. Chem. 2017, 127, 661.
Tandura, S. N.; Shumsky, A. N.; Litvin, E. F.; Kozlova, L. M.; Shuvalova, E. V.; Sharf, V. Z.; Kolesnikov, S. P. Russ. Chem. Bull., Int. Ed. 2001, 50, 1014.
Liu, X.; Zu, Y.; Fu, Y.; Yao, L.; Gu, C.; Wang, W.; Efferth, T. Eur. Food Res. Technol. 2009, 229, 247.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(6), 793–799
Electronic supplementary material
ESM 1
(PDF 4831 kb)
Rights and permissions
About this article
Cite this article
Shyyka, O.Y., Pokhodylo, N.T., Palchykov, V.A. et al. Cage-Like Amines in the Green Protocol of Transannular Thieno[2,3-d]Pyrimidinone Formation as Promising Anticancer Agents. Chem Heterocycl Comp 56, 793–799 (2020). https://doi.org/10.1007/s10593-020-02732-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02732-2