Diagnostic Performance of Novel Urine-Based mRNA Tests (Xpert and Urinary Metabolomics Markers Assay) for Bladder Cancer Detection in Patients with Hematuria
Abstract
BACKGROUND:
Hematuria is the most frequent presenting symptom in the vast majority of bladder cancer (BC) patients. The current recommended evaluation of hematuria includes cross sectional imaging and cystoscopy with possible high negative results, expensive costs and substantial patient burden.
OBJECTIVES:
To validate novel urine-based mRNA-dependant tests; Xpert test and urinary metabolomics assay (CRAT and SLC 25A20genes expression) for BC detection in patients with hematuria.
METHODS:
Patients presented with hematuria to our tertiary care hospital were evaluated by CT urogram and office white light cystoscopy with subsequent inpatient biopsy for positive findings. Voided precystoscopy urine samples were prospectively collected. Xpert test, assay of targeted urinary metabolomics and cytology, were performed. The tests characteristics presumably were calculated based on the ability to identify BC noninvasively.
RESULTS:
Between March 2018 and June 2019, 181 patients were included in the final analysis with mean (±SD) age 62 (±10) years with 168 (92.8%) males. Macroscopic hematuria was encountered in 153 (84.5%) patients with irritative bladder symptoms in 48 (26.5%) patients. BC was confirmed by cystoscopy/biopsy in 36 (19.9%) patients. The performance characteristics of Xpert alone (SN: 73%, SP: 83%, NPV: 92%, PPV: 52%) (AUC 0.84, 95% CI 0.75–0.93, p = 0.001), metabolomics assay alone (SN: 89%, SP: 93%, NPV: 97%, PPV: 78%) (AUC 0.91, 95% CI 0.85–0.98, p < 0.001) and combination of both test results (SN: 66%, SP: 98%, NPV: 92%, PPV: 97%) (AUC 0.83, 95% CI 0.74–0.93, p = 0.001) were notably superior to urine cytology (SN: 30%, SP: 84%, NPV: 83%, PPV: 33%) (AUC 0.58, 95% CI 0.47–0.69, p = 0.154) for BC prediction. Cystoscopy-negative patients (CNP) were followed-up for a median (range) 12 (2–19) months. Re-cystoscopy was done for 35 patients with persistent symptoms. BC was diagnosed in 6 patients. Xpert and urinary metabolomics results were observably positive in those 6 patients.
CONCLUSION:
Xpert test and assay of urinary metabolomics (CRAT and SLC 25A20 genes expression) have the potential for BC detection in hematuria patients. These non invasive urine based tests can help prioritization of the use of invasive diagnostic tests in systems with long waiting times.
ABBREVIATIONS
BC | Bladder cancer |
CNP | Cystoscopy-negative patients |
HG | High-grade |
LG | Low-grade |
NMIBC | Non muscle invasive bladder cancer |
NPV | Negative predictive value |
PPV | Positive predictive value |
SN | Sensitivity |
SP | Specificity |
TURBT | Transurethral resection of bladder tumor |
WLC | White light cystoscopy |
INTRODUCTION
Hematuria is the presenting symptom in the vast majority of bladder cancer (BC) patients [1]. The incidence of urological malignancies, primarily BC, diagnosed after work up for hematuria varies from 2–5% in the setting of persistent asymptomatic microscopic hematuria (AMH) in referred populations [2], reaching up to 10–20% in those with macroscopic hematuria [3]. Prompt evaluation of hematuria can lead to earlier BC diagnosis with possible improved survival [4].
Most guidelines strongly recommend computed tomography (CT) and diagnostic cystoscopy in patients with macroscopic hematuria and selected cases with AMH [5, 6]. Despite its high diagnostic performance, these tools (CT urogram and cystoscopy) are low yield (in cases of AMH-can miss small lesions), expensive and painful procedure [7].
These aforementioned concerns call for rethinking the diagnostic strategy and to base the evaluation of patients based on risk factors such as age, gender, smoking history or carcinogen exposure [8, 9]. Therefore, recent research has focused on biomarkers that can reliably predict which patients with hematuria are at high risk for BC [10].
Nowadays, many biomarkers exist for BC detection [10]. Based on the target of their assessment, these markers include soluble antigens (BTA-Stat, BTA/TRAK, NMP-22, BCLA-4, survivin), cellular morphology (urine cytology) and cell surface antigens (Ucyt, UroVysion and cytokeratins) [10]. However, most of these tests are associated with significant limitations, namely, low sensitivity (SN), specificity (SP), negative predictive value (NPV) and lacking the capacity for prediction of future risk of BC [11].
Recently, genomic markers detection tests as Cxbladder (measures five gene expression; IGF, HOXA, MDK, CDC and IL8R) were investigated as a potential detection modality of urothelial BC that can help triage full urological work up in patients with hematuria [12].
Xpert test is a novel mRNA-based urine test that measures five mRNA targets (CRH, IGF2, ANXA10, ABL1 and UPK1B) in urine. Previous studies had investigated the feasibility and performance of Xpert for surveillance of non muscle invasive bladder cancer (NMIBC) [13, 14]. It demonstrated a high SN and NPV in relation to urine cytology.
On the other hand, metabolic dysfunction has been implicated in a wide variety of human diseases including BC [15]. Division of tumor cells is associated with an increased activity of a variety of metabolic pathways. Significant alterations in the carnitine-acylcarnitine metabolic pathways were detected in urine specimens from BC patients compared to those of healthy controls [16]. The expression of six tissue mRNA genes involved in the carnitine-acylcarnitine metabolic pathway (CPT1A, CPT1B, CPT1C, CPT2, SLC25A20, and CRAT) was assessed by Won and colleagues. CRAT and SLC25A20 were found to be significantly down regulated in BC patients [17].
In this context, we prospectively assessed the performance of Xpert test and urinary metabolomics assay (mRNA genes expression CRAT and SLC 25A20) for BC detection in patients with hematuria.
DESIGN, SETTINGS, AND PARTICIPANTS
Study population
After approval of the Institutional Review Board (ID: 18.02.140), patients with hematuria (macroscopic or microscopic) were assessed for eligibility to this prospective study. Patients who met these criteria were asked to participate in this trial and were included after providing an informed consent form.
Inclusion and exclusion criteria
Inclusion criteria included patients with gross hematuria (single or recurrent attacks) or microscopic hematuria (persistent AMH or symptomatic with irritative bladder symptoms) within 4 weeks of enrollment. Exclusion criteria involved patients who had history of BC, pelvic irradiation, coagulopathy or receiving anticoagulants and patients with upper tract neoplasm or urolithiasis detected by CT urogram.
Urine cytology, Xpert test and urinary metabolomics evaluation
Urine cytology evaluation
Precystoscopy voided urine samples were collected form study participants and evaluated according to the Paris classification system [18]. Suspicious and malignant samples were considered positive results, while, hyperplastic and negative samples for malignancy were considered negative results.
Xpert test
Precystoscopy voided urine sample was added to an equal volume of GeneXpert Urine Transport Reagent within 1 hour of specimen collection. Transferring 4 ml to Xpert cartridge and inserting the cartridge into the GeneXpert instrument were done. In the cartridge the urine cells were filter captured and lased by sonication. The released nucleic acid was eluted and mixed with dry RT-qPCR reagents, and the solution was transferred to the reaction tube for RT-qPCR and detection [19].
Samples were tested to measure5 targets, including ABL1, CRH, IGF2, ANXA10, and UPK1B. ABL1 served as a sample adequacy control for each assay to ensure sufficient assay input.
Assay of urinary metabolite biomarkers (genes expression; CRAT and SLC25A20)
RNA was purified with the selected RNA extraction kits: Norgen Total RNA kit, RNA extraction was performed following the manufacturer’s instructions, the urine samples was incubated with the Norgen slurry resin and after sedimentation the liquid supernatant was eliminated and the resin-containing volume will be preserved with the preservation solution. Then the RNA was reverse transcript by a total volume of 50μl containing 25μl From 1× TaqMan® Universal PCR with 25μl from 20× TaqMan® Gene Expression Assay Mix and 22.5μl of cDNA diluted in RNase-free water. The real-time PCR conditions was as follows: 1 cycle for 20 seconds (sec) at 96°C, followed by 40 cycles of 2 sec at 96°C for denaturation, 15 sec at 60°C for annealing, and 15 sec at 72°C for extension.
The melting program was performed at 72–95°C with a heating rate of 1°C per 45 sec. Data analysis will be carried out using step one plus RT-PCR, using the equation 2-ΔΔ ct.Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was analyzed as an endogenous RNA reference gene and gene expression was normalized to the expression of GAPDH. The primers used for gene amplification are shown in (Supplement table). Samples from 50 control healthy volunteers were retrieved and assessed for CRAT and SLC 25A20 in the same way. Comparison of results are illustrated in (Supplement table B) showing a significant down regulation in BC patient (p < 0.001).
Table 1
Patients demographics
| Patients characteristics | |
| Mean age in years (±SD) | 62 (±10) |
| Sex n (% ) | |
| •Male | 168 (92.8) |
| •Female | 13 (7.2) |
| Hematuria type n (% ) | |
| •Macroscopic | 153 (84.5) |
| •Microscopic | 28 (15.5) |
| ∘ Asymptomatic | 11 (6) |
| ∘ Symptomatic | 17 (9.5) |
| Diabetes mellitus n (% ) | |
| •No | 143 (79) |
| •Yes | 38 (21) |
| Concurrent irritative bladder symptoms n (% ) | |
| •No | 133 (73.5) |
| •Yes | 48 (26.5) |
| History of urological disorder n (% ) | |
| •No | 131 (72.4) |
| •Yes | 50 (27.6) |
| ∘ Urolithiasis | 15 (8.3) |
| ∘ BOO | 22 (12) |
| ∘ UTI | 13 (7.1) |
| Patients with chronic indwelling bladder FB n (% ) | |
| •No | 163 (90) |
| •Yes | 18 (10) |
| ∘ Catheter | 11 (6) |
| ∘ Bladder stone | 7 (4) |
| Smoking history n (% ) | |
| •Never | 31 (17.1) |
| •Former | 92 (50.8) |
| •Current | 58 (32) |
| Cytology result n (% ) | |
| •Negative | 148 (81.8) |
| •Positive | 33 (18.2) |
| Xpert result n (% ) | |
| •Negative | 131 (72.4) |
| •Positive | 50 (27.6) |
| Metabolomics assay n (% ) | |
| •Normal/upregulated | 140 (77.3) |
| •Down regulated | 41 (22.7) |
| Cystoscopy findings n (% ) | |
| •Free | 145 (80.1) |
| •bladder lesion | 36 (19.9) |
| Biopsy findings in positive cystoscopy n (% ) | |
| Stage (TNM) | |
| ∘ Ta, T1, Tis | 30 (83.3) |
| ∘ ≥T2 | 6 (16.7) |
| Grade (WHO/ISUP 2004) | |
| ∘ Low grade | 7 (19.5) |
| ∘ High grade | 29 (80.5) |
UTI, Urinary tract infection; BOO, Bladder outlet obstruction; FB, Foreign body.
Upper tract imaging and office cystoscopy procedures
All study participants were thoroughly evaluated by CT urogram (unless contraindicated; Magnetic resonance imaging was done) to exclude upper tract neoplasm or urolithiasis.
All office cystoscopy procedures were done using flexible white light cystoscopy (WLC). Precise scanning of the bladder was done. Cystoscopy negative patients (CNP) were defined as patients with no gross, suspicious bladder lesions or bloody efflux from ureteral orifices. For all positive or suspicious cystoscopies, biopsy or TURBT was done within 4 weeks from office cystoscopy.
CNP were followed-up consequently according to the predetermined protocol (surveillance telephone call every 3 months and advice for clinic visit for any new hematuria episode). Those with recurrent hematuria, persistent/aggravated irritative bladder symptoms were scheduled for re-cystoscopy. Positive/suspicious re-cystoscopy was evaluated by biopsy to confirm or exclude malignancy.
Outcome measures
The primary outcome included assessment of the diagnostic performance of urine cytology, Xpert test and urinary metabolomics (gene expression CRAT and SLC25A20) for BC detection in patients with hematuria. Subsequently, SN, SP, NPV and positive predictive value (PPV), were determined.
Statistical analysis
All data were computed using IBM statistical software v. 20. Descriptive statistics were reported in terms of number (percentages) or medians (range)/means (±SD) for categorical and continuous variables, respectively. Chi-square and Fisher exact tests are used to detect the association between categorical variables, for the comparison of cytology Xpert monitor, urinary metabolomics as regard to cystoscopy, McNemar test was utilized. Independent samples t-test or Mann-Whitney U-test was used whenever appropriate to compare continuous variables. Cox proportional hazards regression was used for multivariate analysis of hematuria recurrence and BC detection. A critical two-sided P-value <0.05 was used for statistically significant differences.
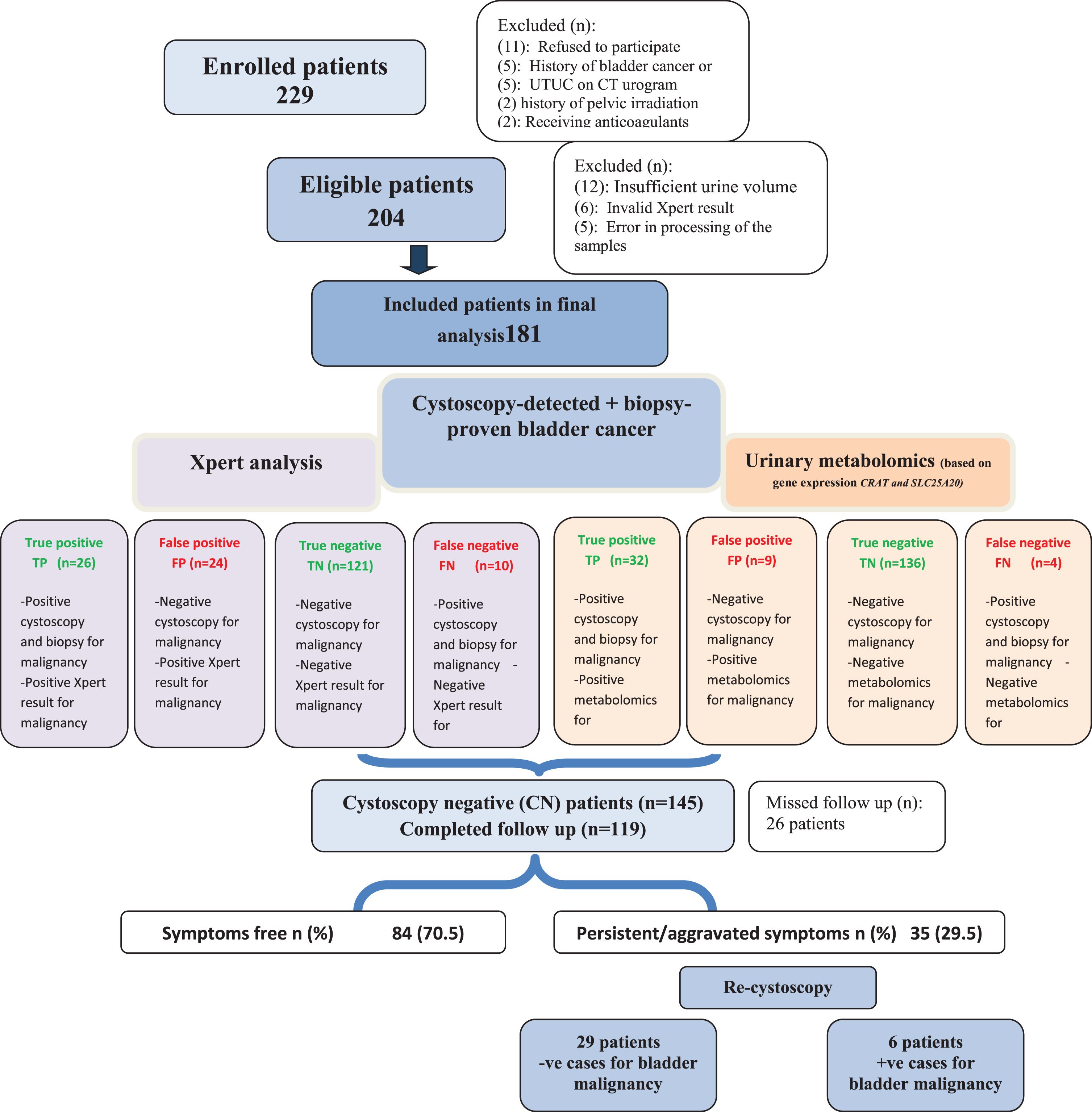
Fig. 1
Study flow chart.
RESULTS
Base line demographics
From March 2018 and June 2019, 204 patients met the study criteria. After exclusion of patients with insufficient urine volume, invalid Xpert results or error in processing of the samples, 181 patients (168 males, 13 females) with a mean (±SD) age of 62(±10) years were included in the analysis of whom 36 (19.9%) patients showed BC on cystoscopy and biopsy. Figure (1) demonstrates the study flow chart while baseline patients’ demographics are illustrated in Table (1).
Urine cytology, Xpert test, urinary metabolomics assay and cystoscopy results
As shown in Table (1), negative cytology was found in 148 (81.8%) and positive cytology in 33 (18.2%) patients. Xpert test and (CRAT and SLC25A20) urinary metabolomics assay came to convey negative results in 131 (72.4%), 129 (71.3%) patients and positive results in 50 (27.6%), 52 (28.7%) patients, respectively.
Outcome measures
The clinical performance characteristics of Xpert monitor, urinary metabolomics assay and urine cytology is clarified in Table (2). The overall SN and SP of Xpert monitor are 73.7% (95% CI: 67–79), 83.4% (95% CI: 79–88), while for urinary metabolomics assay, it is88.9% (95% CI: 85–92), 93.8% (95% CI: 91–96), respectively. The NPV of Xpert test and urinary metabolomics assay are 92.4% (95% CI: 89–96) and 97.1% (95% CI: 85–99), respectively.
Combination of both Xpert and metabolomics assay did not showed an improvement of performance characteristics in terms of SN and NPV (Table 2). On multivariate analysis in Table (3), positive Xpert test and urinary metabolomics assay were independently associated with BC in study participants (p < 0.001).
Table 2
Cross table of Xpert monitor, urinary metabolomics, combination and urine cytology in comparison to cystoscopy result in study participants
| Bladder cancer by cystoscopy and biopsy | Diagnostic characteristics | ||||
| Positive | Negative | Total | % (95% CI) | ||
| Xpert monitor alone | |||||
| Positive Count | 26 | 24 | 50 | SN | 73.7% (95% CI: 67–79) |
| Negative Count | 10 | 121 | 131 | SP | 83.4% (95% CI: 79–88) |
| Total Count | 36 | 145 | 181 | PPV | 52% (95% CI: 24–37) |
| NPV | 92.4% (95% CI: 89–96) | ||||
| Urinary metabolomics (gene expression CRAT, SLC25A20) alone | SN | 88.9% (95% CI: 85–92) | |||
| Down regulated Count | 32 | 9 | 41 | SP | 93.8% (95% CI: 91–96) |
| Normal/Upregulated Count | 4 | 136 | 140 | PPV | 78% (95% CI: 75–81) |
| Total Count | 36 | 145 | 181 | NPV | 97.1% (95% CI: 95–99) |
| Combination of Xpert and metabolomics assay | SN | 66.7% (95% CI: 63–70) | |||
| Positive Count | 22 | 2 | 24 | SP | 98% (95% CI: 97–100) |
| Negative Count | 14 | 143 | 157 | PPV | 97.6% (95% CI: 95–99) |
| Total Count | 33 | 145 | 181 | NPV | 92.8% (95% CI: 89–95) |
| Urine cytology | |||||
| Positive Count | 11 | 22 | 33 | SN | 30.6% (95% CI: 27–44) |
| Negative Count | 25 | 123 | 148 | SP | 84.8% (95% CI: 79–88) |
| Total Count | 36 | 145 | 181 | PPV | 33.6% (95% CI: 30–37) |
| NPV | 83.1% (95% CI: 80–86) | ||||
CRAT, carnitine O-acetyltransferase; SLC25A20, solute carrier family 25 [carnitine/acylcarnitine translocase (CACT)]; SN, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value.
Table 3
Univariate and multivariate analyses for predictors of positive cystoscopy for bladder malignancy
| Patient and tumor characteristics | Bladder cancer by cystoscopy and biopsy | Univariate analysis | Multivariate analysis | |
| HR(95% CI) p value | HR (95% CI) p value | |||
| No | Yes | |||
| N (%) | N (%) | |||
| Mean age in years (±SD) | 63.1 (1.1) | 58 (2.1) | 1.01 (0.9–1.03) 0.47 | |
| Gender n (%) | 1.7 (1.13–2.54) 0.29 | |||
| •Male | 136 (93.8) | 34 (88.9) | ||
| •Female | 9 (6.2) | 4 (11.1) | ||
| Hematuria type n (%) | 1.4 (0.95–1.85) 0.8 | |||
| •Macroscopic | 123 (84.8) | 30 (83.3) | ||
| •Microscopic | 22 (15.2) | 6 (16.7) | ||
| Concurrent irritative bladder symptoms n (%) | 2.2 (1.48–3.30)<0.001 | 1.9 (1.54–2.44)<0.001 | ||
| •No | 122 (84.1) | 11 (30.5) | ||
| •Yes | 23 (15.9) | 25 (69.5) | ||
| History of urological disorder n (%) | ||||
| •No | 107 (73.8) | 24 (66.7) | 1.12 (4.6–1.98) 0.41 | |
| •Yes | 38 (26.2) | 12 (33.3) | ||
| Patients with chronic indwelling bladder FB n (%) | 1.15 (0.68-1.94) 0.77 | |||
| •No | 131 (90.3) | 32 (88.9) | ||
| •Yes | 14 (9.7) | 4 (11.1) | ||
| Smoking status n (%) | 1.4 (0.87–2.13) 0.77 | |||
| •Never | 26 (17.9) | 5 (13.9) | ||
| •Former | 72 (49.7) | 20 (55.6) | ||
| •Current | 47 (32.4) | 11 (30.5) | ||
| Cytology result n (%) | 1.17 (0.74–0.1.64) 0.55 | |||
| •tNegative | 123 (84.8) | 25 (69.5) | ||
| •Positive | 22 (15.2) | 11 (30.5) | ||
| Xpert result n (%) | 2.38 (1.74–2.95)<0.001 | 2.1 (1.78–2.54) 0.006 | ||
| •Negative | 121 (83.4) | 10 (27.8) | ||
| •Positive | 24 (16.6) | 26 (72.2) | ||
| Metabolomics result n (%) | 2.47 (1.84–3.12)<0.001 | 2.24 (1.81–2.71) 0.001 | ||
| •Normal | 118 (81.4) | 11 (30.5) | ||
| •down regulated | 27 (18.2) | 25 (69.5) | ||
| Combination of Xpert and Metabolomics results n (%) | 2.41 (1.79–3.02)<0.001 | 2.19 (1.74–2.49) 0.003 | ||
| •Negative | 143 (98.6) | 14 (38.9) | ||
| •Positive | 2 (1.4) | 22 (61.1) | ||
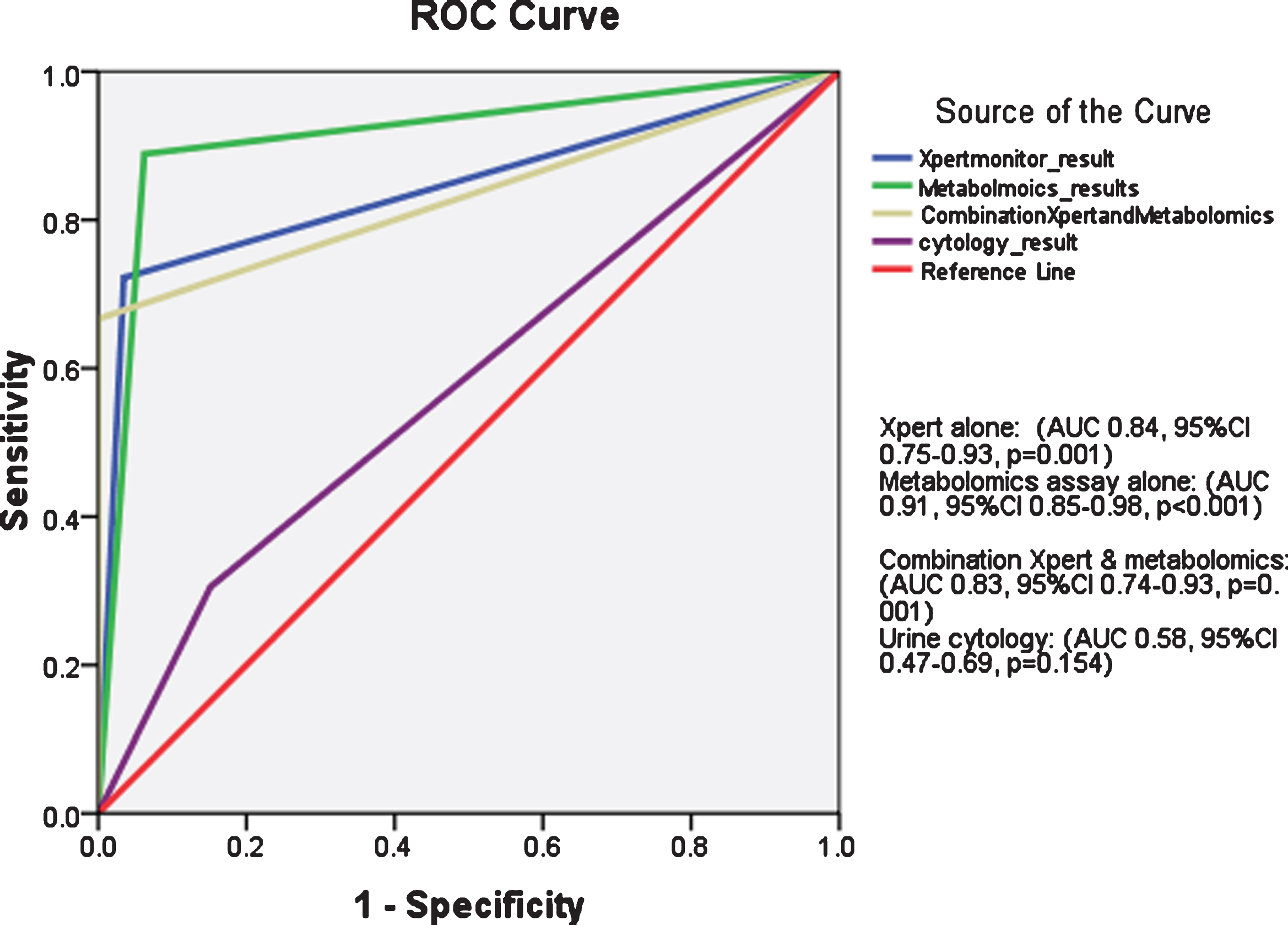
As shown in Fig. (2), receiver operator characteristics (ROC) curve showed superiority of Xpert alone, metabolomics assays alone or combination of both tests for BC prediction as compared to urine cytology.
CNP (145) were monitored by regular cystoscopy according to the predetermined protocols for a median (range) period of 12 (2–19) months. Out of 119 patients who completed the follow up, persistent /aggravated symptoms (hematuria or irritative bladder symptoms) were encountered in 35 (29.5%) patients. Re-cystoscopy was repeated in those patients, showing BC in 6patients. The pathology in all of these 6 patients were NMIBC (Ta/1T1 in 5 patients and CIS in 1 patient) while the tumor grade was low and high grade in 4 and 2 patients, respectively. Notably, Xpert test and (CRAT and SLC25A20) urinary metabolomics assays were positive in these 6 patients.
DISCUSSION
Hematuria is a considerable health problem that can imply serious underlying pathology with an incidence of urinary tract malignancies in approximately 2–5% in patients with microscopic hematuria [20] and 10–20% in those with macroscopic hematuria [21]. Most of the available recommendations for investigating a patient with hematuria follow much the same way. As a first step, a detailed history is taken and search for glomerular disease is exhausted. Further urological evaluation with imaging of the upper tract and cystoscopy is recommended on the basis of further risk calculation (Age, macroscopic hematuria, smoking history) [22].
These invasive diagnostic tools (CT and cystoscopy) are limited by its financial cost, emotional impact and potential adverse events [23, 24]. In addition, possible delay between initial presentation and scheduling of these diagnostic procedures may negatively influence the outcomes for BC patients [25]. The introduction of such a non invasive test that can triage patients with hematuria, might be a helpful step in improving practice among those peculiar group of patients (prioritization for invasive work up).
Urine cytology is a non-invasive adjunct to cystoscopy. It has a high SN for detecting high-grade (HG) urothelial BC, but SN for detection of low-grade (LG) tumors ranged from only 4 to 31% [26]. Furthermore, the accuracy of cytology is pathological-dependent and is thus not of high quality in all places. Other urine-based markers (BTA stat, BTA TRACK, Survivin) and cell-based (Urovysion-uCyt+™, DD23) assays were evaluated over the last decades to assess its performance characteristics for BC detection [27].
In this study, we evaluated the diagnostic performance characteristics of two novel urine-based RNA tests; Xpert and urinary metabolomics assay (gene expression CRAT and SLC25A20) as a preliminary step for triage patients with hematuria. In addition, the performance of voided urine cytology was assessed in our cohort.
Xpert showed a higher SN (73% vs. 30%) and NPV (92% vs. 83%) when compared to urine cytology. On the other hand, the SP of Xpert was slightly lower than cytology (83% vs. 84%). Our results go hand in hand with the findings of Valenberg et al. which showed a higher SN (74%) and NPV (93%) of Xpert [14]. Valenberg and his co workers demonstrated a higher NPV (98%) for HG tumors. In subgroup analysis of our cohort, NPV for HG tumors reached 100%. These matched findings confirm the fact that negative Xpert allows to consistently exclude BC.
In LG tumors, Xpert demonstrated a high SN when compared to urine cytology (71% vs. 21%). This outcome matches with the results of Pichler et al., which showed a maintained high SN of Xpert in LG tumors (77%) [13]. These -in line-findings confirm the superiority of Xpert over urine cytology in LG tumors, and, provide a reliable non invasive method for BC detection especially in those set of patients (low and intermediate risk NMIBC).
Similarly, urinary metabolomics assays in our study for BC detection achieved superior performance when compared to cytology (SN: 88% vs. 30% and NPV: 97% vs. 83%). Notably, these diagnostic characteristics were maintained in LG tumors (SN: 82% and NPV: 95%). The present data generated by the use of urinary metabolomics (mRNA-based gene expression CRAT and SLC25A20), externally validated the previous presented data by Won et al [17].
Fig. 2
Receiver operator characteristics (ROC) curve for Xpert test alone, metabolomics assay alone, combination of both and urine cytology for bladder cancer detection in study cohort.
The need for repeated evaluation in patients with hematuria after an initial negative work-up is questionable. Although the data on which to base recommendations for hematuria follow-up care is limited, consideration may be given to reevaluating any patient with gross hematuria or persistent AMH with a smoking history at two to five years [28].
In our study, 119 initially CNP completed median follow up of 12 months. Re-cystoscopy was needed in 35 (29.5%) patients, of whom, 6 (5%) patients showed BC. Notably, Xpert and urinary metabolomics assays were positive in those 6 patients. This can imply that these tests may have a predictive role to consider more helpful diagnostic tools as blue light cystoscopy or close surveillance for initial CNP with positive Xpert or urinary metabolomics assay; however, larger sample studies with long follow up are warranted to check this point.
These promising results of Xpert and urinary metabolomics assays (high SN and NPV, possible predictive capacity) may be additive tools for risk categorization of patients with hematuria, in addition to other risk factors (smoking, occupational exposure), to triage patients who are prior to invasive cystoscopy and CT. Moreover, Xpert is an easy computerized test, does not an expert lab or investigator to do. These criteria alleviate the impact of training status and experience of the laboratory staff on test performance.
Our work is the first study to assess the performance of these urine-based mRNA tests (Xpert and urinary metabolomics assay) for BC detection in hematuria patients with an average follow-up of CNP. As well, single center nature of our study excluded test handling and execution errors.
Our study is limited by inclusion of low number of positive cases. Lack of follow up urinalysis in CNP is another considerable limitation in our study, however the nature of our institute (referral center covering a wide population area) was the main obstacle in recruiting such clinically asymptomatic patients for urinalysis (Compliance issues). Future prospective multicenter studies are warranted to validate our results for heterogeneous populations and to determine any added value in the use of these tests, costs, misinterpretation, and the emotional stress encountered by both patient and physician in assessing the reliability of these markers before application in ‘routine’ clinical use.
CONCLUSIONS
Xpert test and assay of urinary metabolomics (gene expression CRAT and SLC25A20) have the potential for BC detection in hematuria patients with high performance compared to urine cytology. These non invasive urine based tests can help prioritization the use of invasive diagnostic tests in systems with long waiting times. External validation and cost analysis for wide popularization are warranted.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/BLC-200318.
ACKNOWLEDGMENTS
The authors have no acknowledgment.
FUNDING
This work was funded by Mansoura University competitive projects. Project ID: mu-med-16-13.
AUTHOR CONTRIBUTIONS
AAE: Principal investigator, performed the statistics and wrote the paper; AA: Study design, revised the statistics and the paper; SM: Performed lab procedures and follow up of patients; AEA: Performed lab procedures and follow up of patients; MMY: Study design and revised the paper; HAE: Study design and revised the paper.
ETHICAL CONSIDERATIONS
Trial registration: clinicaltrials.gov ID: NCT04194112.
CONFLICT OF INTEREST
The authors have nothing to declare.
REFERENCES
[1] | Bruyninckx R , Buntinx F , Aertgeerts B , Van Casteren V . The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br J Gen Pract. (2003) ;53: (486):31–5. |
[2] | Gonzalez AN , Lipsky MJ , Li G , Rutman MP , Cooper KL , Weiner DM , et al. The Prevalence of Bladder Cancer During Cystoscopy for Asymptomatic Microscopic Hematuria. Urology. (2019) ;126: :34–8. |
[3] | Ghandour R , Freifeld Y , Singla N , Lotan Y . Evaluation of Hematuria in a Large Public Health Care System. Bladder Cancer. (2019) ;5: (2):119–29. |
[4] | Ramirez D , Gupta A , Canter D , Harrow B , Dobbs RW , Kucherov V , et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int.. (2016) ;117: (5):783–6. |
[5] | Davis R , Jones JS , Barocas DA , Castle EP , Lang EK , Leveillee RJ , et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. (2012) ;188: (6 Suppl):2473–81. |
[6] | Wollin T , Laroche B , Psooy K . Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. (2009) ;3: (1):77–80. |
[7] | Svatek RS , Hollenbeck BK , Holmang S , Lee R , Kim SP , Stenzl A , et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. (2014) ;66: (2):253–62. |
[8] | Subak LL , Grady D . Asymptomatic Microscopic Hematuria-Rethinking the Diagnostic Algorithm. JAMA Intern Med. (2017) ;177: (6):808–9. |
[9] | Burger M , Catto JW , Dalbagni G , Grossman HB , Herr H , Karakiewicz P , et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) ;63: (2):234–41. |
[10] | Shirodkar SP , Lokeshwar VB . Bladder tumor markers: from hematuria to molecular diagnostics–where do we stand? Expert Rev Anticancer Ther. (2008) ;8: (7):1111–23. |
[11] | Tilki D , Burger M , Dalbagni G , Grossman HB , Hakenberg OW , Palou J , et al. Urine markers for detection and surveillance of non-muscle-invasive bladder cancer. Eur Urol. (2011) ;60: (3):484–92. |
[12] | Darling D , Luxmanan C , O’Sullivan P , Lough T , Suttie J . Clinical Utility of Cxbladder for the Diagnosis of Urothelial Carcinoma. Adv Ther. (2017) ;34: (5):1087–96. |
[13] | Pichler R , Fritz J , Tulchiner G , Klinglmair G , Soleiman A , Horninger W , et al. Increased accuracy of a novel mRNA-based urine test for bladder cancer surveillance. BJU Int. (2018) ;121: (1):29–37. |
[14] | Valenberg F , Hiar AM , Wallace E , Bridge JA , Mayne DJ , Beqaj S , et al. Prospective Validation of an mRNA-based Urine Test for Surveillance of Patients with Bladder Cancer. Eur Urol. (2019) ;75: (5):853–60. |
[15] | Huang Z , Lin L , Gao Y , Chen Y , Yan X , Xing J , et al. Bladder cancer determination via two urinary metabolites: a biomarker pattern approach. Mol Cell Proteomics. (2011) ;10: (10):28. |
[16] | Jin X , Yun SJ , Jeong P , Kim IY , Kim WJ , Park S . Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. (2014) ;5: (6):1635–45. |
[17] | Kim WT , Yun SJ , Yan C , Jeong P , Kim YH , Lee IS , et al. Metabolic Pathway Signatures Associated with Urinary Metabolite Biomarkers Differentiate Bladder Cancer Patients from Healthy Controls. Yonsei Med J. (2016) ;57: (4):865–71. |
[18] | Barkan GA , Wojcik EM , Nayar R , Savic-Prince S , Quek ML , Kurtycz DF , et al. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Acta Cytol. (2016) ;60: (3):185–97. |
[19] | Wallace E , Higuchi R , Satya M , McCann L , Sin MLY , Bridge JA , et al. Development of a 90-Minute Integrated Noninvasive Urinary Assay for Bladder Cancer Detection. J Urol. (2018) ;199: (3):655–62. |
[20] | Schmitz-Drager BJ , Kuckuck EC , Zuiverloon TC , Zwarthoff EC , Saltzman A , Srivastava A , et al. Microhematuria assessment an IBCN consensus-Based upon a critical review of current guidelines. Urol Oncol. (2016) ;34: (10):437–51. |
[21] | Bolenz C , Schroppel B , Eisenhardt A , Schmitz-Drager BJ , Grimm MO . The Investigation of Hematuria. Dtsch Arztebl Int. (2018) ;115: (48):801–7. |
[22] | Tan WS , Feber A , Sarpong R , Khetrapal P , Rodney S , Jalil R , et al. Who Should Be Investigated for Haematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients: Eur Urol. (2018) ;74: (1):10–4. doi: 10.1016/j.eururo.2018.03.008. Epub 2018 Apr 10. |
[23] | Stav K , Leibovici D , Goren E , Livshitz A , Siegel YI , Lindner A , et al. Adverse effects of cystoscopy and its impact on patients’ quality of life and sexual performance. Isr Med Assoc J. (2004) ;6: (8):474–8. |
[24] | Blackwell RH , Kirshenbaum EJ , Zapf MAC , Kothari AN , Kuo PC , Flanigan RC , et al. Incidence of Adverse Contrast Reaction Following Nonintravenous Urinary Tract Imaging. Eur Urol Focus. (2017) ;3: (1):89–93. |
[25] | McCombie SP , Bangash HK , Kuan M , Thyer I , Lee F , Hayne D . Delays in the diagnosis and initial treatment of bladder cancer in Western Australia. BJU Int. (2017) ;3: :28–34. |
[26] | Yafi FA , Brimo F , Steinberg J , Aprikian AG , Tanguay S , Kassouf W . Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. (2015) ;33: (2):15. |
[27] | Schmitz-Drager BJ , Droller M , Lokeshwar VB , Lotan Y , Hudson MA , van Rhijn BW , et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: the WHO/ICUD consensus. Urol Int. (2015) ;94: (1):1–24. |
[28] | Pichler R , Heidegger I , Leonhartsberger N , Stohr B , Aigner F , Bektic J , et al. The need for repeated urological evaluation in low-risk patients with microscopic hematuria after negative diagnostic work-up. Anticancer Res. (2013) ;33: (12):5525–30. |



