Abstract
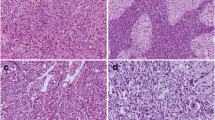
To examine the clinicopathologic and immunohistochemical features of a group of newly defined low-grade oncocytic renal tumors (LOT) that have the “CD117 negative/cytokeratin (CK)7 positive” immunoprofile. We have queried our hospital database and found 4456 consecutive renal tumors between 2016 and 2019. Among these renal tumors, eight (8) cases meet the morphologic and immunohistochemical characterization for low-grade oncocytic renal tumor (LOT). The eight (8) patients’ mean age is 56.6 years (range 39–70 years old), and the male to female ratio is 1:1. Macroscopically, these LOTs generally present with tan-brown and solid cut surfaces and demonstrate similar solid, compact nested growth pattern microscopically. Tumor cells exhibit oncocytic cytoplasm and uniformly rounded to oval nuclei. There are areas of edematous stroma containing dispersed single or small clustered tumor cells. All tumors are negative for CD117 and positive for CK7. Uniform reactivity is also found for BerEP4, cyclin D1, and SDHB. Besides, CD10, vimentin, and AMACR are either negative or only focally positive. All of the tumors are negative for CA9 and TFE. The Ki-67 index is less than 5% in the seven (7) internal cases. Seven (7) of the eight (8) patients who are available for follow-up are alive and without disease recurrence (mean follow-up period of 21.6 months, ranging from 6 to 43 months). We described a group of low-grade oncocytic renal tumors identified retrospectively in a large tertiary cancer center, which was probably the first report originated from China or even Asia in the English literature so far. These tumors demonstrated eosinophilic cytoplasm and low-grade appearing nuclei with a “CD117 negative/CK7 positive” immunoprofile. The incidence rate was about 3.7% of the oncocytic renal tumors and 0.18% of all the renal tumors that were received in our lab during the four-year period. It is necessary to separate this group of tumors by its characteristic morphologic and immunophenotypic features.




Similar content being viewed by others
References
Moch H, Humphrey PA, Ulbright TM, Reuter V (2016) WHO classification of tumours of the urinary system and male genital organs. International Agency for Research on Cancer, Lyon, France
Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, Zhou M, Argani P, ISUP Renal Tumor Panel (2013) The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol 37:1469–1489
Williamson SR, Gadde R, Trpkov K, Hirsch MS, Srigley JR, Reuter VE, Cheng L, Kunju LP, Barod R, Rogers CG, Delahunt B, Hes O, Eble JN, Zhou M, McKenney JK, Martignoni G, Fleming S, Grignon DJ, Moch H, Gupta NS (2017) Diagnostic criteria for oncocytic renal neoplasms: a survey of urologic pathologists. Hum. Pathol. 63:149–156
Iczkowski KA, Czaja RC (2019) Eosinophilic kidney tumors: old and new. Arch Pathol Lab Med. 143(12):1455–1463
Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Gupta S, Hancock SL, Kim JJ, Kuzel TM, Lam ET, Lau C, Levine EG, Lin DW, Michaelson MD, Olencki T, Pili R, Plimack ER, Rampersaud EN, Redman BG, Ryan CJ, Sheinfeld J, Shuch B, Sircar K, Somer B, Wilder RB, Dwyer M, Kumar R (2015) National comprehensive cancer network, Kidney cancer, version 3.2015. J Natl Compr Canc Netw 13:151–159
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, Leibovich BC (2014) Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 32:4059–4065
Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, Derweesh IH, Herrell SD, Hilton S, Jonasch E, Lin DW, Reuter VE, Chang SS (2013) Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 190:407–416
Trpkov K, Hes O (2019) New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology. 74(1):31–59
Siadat F, Trpkov K (2020) ESC, ALK, HOT and LOT: three letter acronyms of emerging renal entities knocking on the door of the WHO classification. Cancers (Basel) 12(1):168. Published 2020 January 9th. https://doi.org/10.3390/cancers12010168
Trpkov K, Williamson SR, Gao Y, Martinek P, Cheng L, Sangoi AR, Yilmaz A, Wang C, San Miguel Fraile P, Perez Montiel DM, Bulimbasić S, Rogala J, Hes O (2019) Low-grade oncocytic tumor of kidney (CD117-negative, cytokeratin 7-positive): a distinct entity? Histopathology. 75(2):174–184
Trpkov K, Yilmaz A, Uzer D, Dishongh KM, Quick CM, Bismar TA, Gokden N (2010) Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology. 57(6):893–906
Abrahams NA, MacLennan GT, Khoury JD et al (2004) Chromophobe renal cell carcinoma: a comparative study of histological, immunohistochemical and ultrastructural features using high throughput tissue microarray. Histopathology. 45:593–602
Hes O, Brunelli M, Michal M, Cossu Rocca P, Hora M, Chilosi M, Mina M, Boudova L, Menestrina F, Martignoni G (2006) Oncocytic papillary renal cell carcinoma: a clinicopathologic, immunohistochemical, ultrastructural, and interphase cytogenetic study of 12 cases. Ann Diagn Pathol. 10(3):133–139
Amin MB, Gupta R, Hes O et al (2009) Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 33(3):393–400
Ellis CL, Eble JN, Subhawong AP, Martignoni G, Zhong M, Ladanyi M, Epstein JI, Netto GJ, Argani P (2014) Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age, and stage. Mod Pathol. 27(6):875–886
Ohe C, Smith SC, Sirohi D, Divatia M, de Peralta-Venturina M, Paner GP, Agaimy A, Amin MB, Argani P, Chen YB, Cheng L, Colecchia M, Compérat E, Werneck da Cunha I, Epstein JI, Gill AJ, Hes O, Hirsch MS, Jochum W, Kunju LP, Maclean F, Magi-Galluzzi C, McKenney JK, Mehra R, Nesi G, Osunkoya AO, Picken MM, Rao P, Reuter VE, de Oliveira Salles PG, Schultz L, Tickoo SK, Tomlins SA, Trpkov K, Amin MB (2018) Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol. 42(3):279–292
Gill AJ, Hes O, Papathomas T, Šedivcová M, Tan PH, Agaimy A, Andresen PA, Kedziora A, Clarkson A, Toon CW, Sioson L, Watson N, Chou A, Paik J, Clifton-Bligh RJ, Robinson BG, Benn DE, Hills K, Maclean F, Niemeijer ND, Vlatkovic L, Hartmann A, Corssmit EPM, van Leenders GJLH, Przybycin C, McKenney JK, Magi-Galluzzi C, Yilmaz A, Yu D, Nicoll KD, Yong JL, Sibony M, Yakirevich E, Fleming S, Chow CW, Miettinen M, Michal M, Trpkov K (2014) Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 38(12):1588–1602
Klein MJ, Valensi QJ (1976) Proximal tubular adenomas of kidney with so-called oncocytic features. A clinicopathologic study of 13 cases of a rarely reported neoplasm. Cancer 38:906–914
Kravtsov O, Gupta S, Cheville J, Hernandez L, Jimenez R (2020) Low-grade oncocytic tumor of kidney (CK7-positive, CD117-negative): a single institutional experience with incidence and clinicopathological characteristics. Modern Pathology 33:917–918 Abstract number 950
Thoenes W, Storkel S, Rumpelt HJ (1985) Human chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol 48:207–217
Kuroda N, Tanaka A, Yamaguchi T, Kasahara K, Naruse K, Yamada Y, Hatanaka K, Shinohara N, Nagashima Y, Mikami S, Oya M, Hamashima T, Michal M, Hes O (2013) Chromophobe renal cell carcinoma, oncocytic variant: a proposal of a new variant giving a critical diagnostic pitfall in diagnosing renal oncocytic tumors. Med Mol Morphol. 46(1):49–55
Ng KL, Rajandram R, Morais C, Yap NY, Samaratunga H, Gobe GC, Wood ST (2014) Differentiation of oncocytoma from chromophobe renal cell carcinoma (RCC): can novel molecular biomarkers help solve an old problem? J Clin Pathol. 67(2):97–104
Morini A, Drossart T, Timsit M, Mejean A, Thibault C, Gimenez-Roqueplo A, Favier F, Burnichon N, Verkarre V. Immunohistochemical evaluation of the mTOR pathway of genetically characterized chromophobe renal cell carcinomas: a pilot study of 20 cases. Modern Pathology. volume 33, pages 940–941(2020). Abstract number 976
Delongchamps NB, Galmmiche L, Eiss D, et al. Hybrid tumor “oncocytoma-chromophobe renal cell carcinoma” of the kidney: a report of seven sporadic cases. BJU Int. 2009;103(10):1381–1384, Hybrid tumour ‘oncocytoma-chromophobe renal cell carcinoma’ of the kidney: a report of seven sporadic cases.
Petersson F, Gatalica Z, Grossmann P, Perez Montiel MD, Alvarado Cabrero I, Bulimbasic S, Swatek A, Straka L, Tichy T, Hora M, Kuroda N, Legendre B, Michal M, Hes O (2010) Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch 456:355–365
Ruiz-Cordero R, Rao P, Li L, Qi Y, Atherton D, Peng B, Singh RR, Kim TB, Kawakami F, Routbort MJ, Alouch N, Chow CWB, Tang X, Lu W, Brimo F, Matin SF, Wood CG, Tannir NM, Wistuba II, Chen K, Wang J, Medeiros LJ, Karam JA, Tamboli P, Sircar K (2019) Hybrid oncocytic/chromophobe renal tumors are molecularly distinct from oncocytoma and chromophobe renal cell carcinoma. Mod Pathol. 32(11):1698–1707
Mikami S, Kuroda N, Nagashima Y, Ohe C, Hayashi H, Mizuno R, Oya M, Kameyama K (2019) Classification of solid renal tumor with oncocytic/eosinophilic cytoplasm: is hybrid oncocytic/chromophobe renal tumor a subtype of oncocytoma, chromophobe renal cell carcinoma, or a distinct tumor entity? Ann Transl Med. 7(Suppl 8):S350
Acknowledgments
This work was supported by the Tianjin Medical University Cancer Institute and Hospital and National Clinical Research Center for Cancer.
Funding
This work was funded by the Project the National Natural Science Foundation of China (No. 81700103), the Tianjin Municipal Health Bureau Science and Technology Foundation (Grant No.16KG125), and the Project of Tumor Translational Medicine Seed Fund of Tianjin Medical University Cancer Institute and Hospital (No. 1905).
Author information
Authors and Affiliations
Contributions
All the authors met the criteria listed in the ICMJE recommendations for the qualification of authorship.
Qianru Guo, Ning Liu, Frank Wang, Bo Yang, Lisha Qi, Cheng Wang, and Wenfeng Cao designed the research study.
Qianru Guo, Ning Liu, Frank Wang, Bo Yang, Yong Wang, Wenshuai Zhang, Qiujuan Huang, Wei Zhao, Changxu Liu, Tongyuan Qu, Lingmei Li, Yanan Gao, Lu Cao, Danyang Ren, Bin Meng, Lisha Qi, Cheng Wang, and Wenfeng Cao performed the research.
Yuhong Guo, Zi Cao, and Yalei Wang contributed essential reagents or tools.
Qianru Guo, Ning Liu, Frank Wang, Wenshuai Zhang, Cheng Wang, and Wenfeng Cao analyzed the data.
Qianru Guo, Ning Liu, Frank Wang, Cheng Wang, and Wenfeng Cao wrote the paper.
Corresponding authors
Ethics declarations
This study protocol was approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (bc2016022).
Conflict of interest
The authors declare that they have no financial or other conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Guo, Q., Liu, N., Wang, F. et al. Characterization of a distinct low-grade oncocytic renal tumor (CD117-negative and cytokeratin 7-positive) based on a tertiary oncology center experience: the new evidence from China. Virchows Arch 478, 449–458 (2021). https://doi.org/10.1007/s00428-020-02927-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02927-0