Abstract
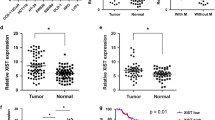
The long non‐coding RNA antisense 1 ADAMTS9-AS1 has been reported to predict the survival in several tumors, including bladder cancer and breast cancer. However, the clinical significance and biological behaviors of ADAMTS9-AS1 in colorectal cancer (CRC) have not been reported yet. In this study, the expression of ADAMTS9-AS1 was measured in CRC tissues and cell lines using quantitative real-time PCR analysis. The clinical significance of ADAMTS9-AS1 was evaluated with Chi-squared test, Kaplan–Meier method and Cox regression analysis in CRC patients. CCK8 assay, colony formation assay, flow cytometry and transwell assay were used to explore the biological function of ADAMTS9-AS1 knockdown in CRC cell lines (SW1116 and HT29). We further explore the role of ADAMTS9-AS1 in vivo though xenograft tumor assay. Our data showed that ADAMTS9-AS1 expression level was significantly up-regulated in CRC tissues and cell lines compared with corresponding controls. High ADAMTS9-AS1 level was associated with TNM stage, lymph node invasion and worse survival prognosis. Depletion of ADAMTS9-AS1 significantly suppressed cell proliferation, G1/S transition, migration and invasion, as well as suppressed CDK4/Cyclin D1 and epithelial–mesenchymal transition (EMT). To sum up, these findings illustrated that ADAMTS9-AS1 might be a promising therapeutic target and prognostic factor for CRC.





Similar content being viewed by others
Abbreviations
- CRC:
-
Colorectal cancer
- lncRNAs:
-
Long non-coding RNAs
- EMT:
-
Epithelial to mesenchymal transition
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- NC:
-
Negative control
- CCK-8:
-
Cell Counting Kit-8
- PVDF:
-
Polyvinylidene fluoride
References
Siegel RL, Miller KD, Jemal A. Cancer statistics 2019 CA. Cancer J Clin. 2019;69:7–34.
Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765.
Zhao L, Zhang D, Shen Q, et al. KIAA1199 promotes metastasis of colorectal cancer cells via microtubule destabilization regulated by a PP2A/stathmin pathway. Oncogene. 2019;38:935–49.
Chibaudel B, Tournigand C, Bonnetain F, et al. Therapeutic strategy in unresectable metastatic colorectal cancer: an updated review. Therap Adv Med Oncol. 2015;7:153–69.
Jiang C, Li X, Zhao H, Liu H. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer. 2016;15:62.
Wu C, Zhu XT, Xia L, et al. High expression of long noncoding RNA PCNA-AS1 promotes non-small-cell lung cancer cell proliferation and oncogenic activity via upregulating CCND1. J Cancer. 2020;11:1959–67.
Liu T, Han Z, Li H, Zhu Y, Sun Z, Zhu A. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol Cancer. 2018;17:118.
Liang C, Zhao T, Li H, et al. Long non-coding RNA ITIH4-AS1 accelerates the proliferation and metastasis of colorectal cancer by activating JAK/STAT3 signaling. Mol Ther Nucleic Acids. 2019;18:183–93.
Zhang J, Bian Z, Jin G, et al. Long non-coding RNA IQCJ-SCHIP1 antisense RNA 1 is downregulated in colorectal cancer and inhibits cell proliferation. Ann Transl Med. 2019;7:198.
Wang H, Fu Z, Dai C, et al. LncRNAs expression profiling in normal ovary, benign ovarian cyst and malignant epithelial ovarian cancer. Sci Rep. 2016;6:38983.
Li Z, Yao Q, Zhao S, Wang Y, Li Y, Wang Z. Comprehensive analysis of differential co-expression patterns reveal transcriptional dysregulation mechanism and identify novel prognostic lncRNAs in esophageal squamous cell carcinoma. OncoTargets Ther. 2017;10:3095–105.
Zhu N, Hou J, Wu Y, et al. Integrated analysis of a competing endogenous RNA network reveals key lncRNAs as potential prognostic biomarkers for human bladder cancer. Medicine. 2018;97:e11887.
Fan CN, Ma L, Liu N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J Transl Med. 2018;16:264.
Xing Y, Zhao Z, Zhu Y, Zhao L, Zhu A, Piao D. Comprehensive analysis of differential expression profiles of mRNAs and lncRNAs and identification of a 14-lncRNA prognostic signature for patients with colon adenocarcinoma. Oncol Rep. 2018;39:2365–75.
Wang J, Zhang C, Wu Y, He W, Gou X. Identification and analysis of long non-coding RNA related miRNA sponge regulatory network in bladder urothelial carcinoma. Cancer Cell Int. 2019;19:327.
Wan J, Jiang S, Jiang Y, et al. Data mining and expression analysis of differential lncRNA ADAMTS9-AS1 in prostate cancer. Front Genet. 2019;10:1377.
Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–64.
Goel S, DeCristo MJ, McAllister SS, Zhao JJ. CDK4/6 Inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol. 2018;28:911–25.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer. 2011;11:558–72.
Ding J, Xu K, Sun S, et al. SOCS1 blocks G1-S transition in hepatocellular carcinoma by reducing the stability of the CyclinD1/CDK4 complex in the nucleus. Aging (Albany NY). 2020;12:3962–75.
Tang J, Huang F, Wang H, et al. Knockdown of TPT1-AS1 inhibits cell proliferation, cell cycle G1/S transition, and epithelial-mesenchymal transition in gastric cancer. Bosn J Basic Med Sci. 2020. https://doi.org/10.17305/bjbms.2020.4470.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95.
Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420–8.
Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36.
Funding
This work was funded by 2019 Provincial Key Laboratory of Medical Physics and Technology Safety and Security Open Fund (no. LMPT201908).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
The present study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The Second Hospital of Anhui Medical University (Approval number: AM3-289; 2015.10.15, Anhui, China).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, W., Tu, Q., Yu, L. et al. LncRNA ADAMTS9-AS1, as prognostic marker, promotes cell proliferation and EMT in colorectal cancer. Human Cell 33, 1133–1141 (2020). https://doi.org/10.1007/s13577-020-00388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00388-w