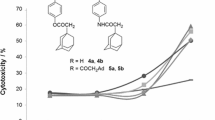
Cytotoxicities of the quinolizidine alkaloid (–)-cytisine and 19 of its derivatives with substituents in the 3-, 9-, and 11-positions were assessed against A431 (epidermal carcinoma), A375 (melanoma), and HCT 116 (colorectal carcinoma) tumor cell lines using the MTT assay (etoposide reference drug). Practically all synthesized compounds at a concentration of 30 μM possessed slight ability to inhibit metabolic activity of these cell lines except benzylcytisine 4, methylcytisines 18 and 19, which contained a phenylurea fragment in the 9- or 11-position of the 2-pyridone core, and 11-chloro adamantylthiocarboxamide 16. Thiocarboxamide 16 reduced A431 cell survival up to 56.06% under the experimental conditions; derivatives 4, 18, and 19, of HCT 116 cell line by 57.52, 58.84, and 56.34%, respectively.
Similar content being viewed by others
References
D. J. Newman and G. M. Cragg, J. Nat. Prod., 79, 629 (2016).
K. Mohan, R. Jeyachandran, and R. Deepa, Ann. Phytomed., 1, 46 (2012).
Z. Habli, G. Toumieh, M. Fatfat, O. N. Rahal, and H. Gali-Muhtasi, Molecules, 22, 250 (2017).
I. P. Tsypysheva, P. R. Petrova, A. V. Koval′skaya, N. Lobov, M. A. Maksimova, L. F. Zainullina, V. I. Vinogradova, V. A. Vakhitov, Yu. V. Vakhitova, and F. Z. Galin, Chem. Nat. Compd., 54, 938 (2018).
I. P. Tsypysheva, A. V. Koval′skaya, P. R. Petrova, A. N. Lobov, A. S. Erastov, Z. R. Zileeva, V. A. Vakhitov, and Yu. V. Vakhitova, Tetrahedron, 76 (7), 130902 (2020).
H.-Q. Li, P.-C. Lv, T. Yan, and H.-L. Zhu, Anti-Cancer Agents Med. Chem., 9, 471 (2009).
V. Kumar and S. S. Chimni, Anti-Cancer Agents Med. Chem., 15, 163 (2015).
C. C. Boido and F. Sparatore, Farmaco, 54, 438 (1999).
I. P. Tsypysheva, A. V. Koval′skaya, N. S. Makara, A. N. Lobov, I. A. Petrenko, E. G. Galkin, T. A. Sapozhnikova, F. S. Zarudii, and M. S. Yunusov, Chem. Nat. Compd., 48, 629 (2012).
F. Moll and G. Luputiu, Arch. Pharm., 305, 771 (1972).
I. P. Tsypysheva, A. V. Koval′skaya, A. N. Lobov, M. Kh. Salimgareeva, U. Sh. Fatkullina, P. R. Petrova, S. F. Gabdrakhmanova, N. S. Makara, K. Yu. Suponitskii, Yu. V. Vakhitova, F. S. Zarudii, and M. S. Yunusov, Chem. Nat. Compd., 49, 707 (2013).
V. A. Fedorova, R. A. Kadyrova, A. V. Slita, A. A. Muryleva, P. R. Petrova, A. V. Kovalskaya, A. N. Lobov, D. O. Tsypyshev, S. S. Borisevich, I. P. Tsypysheva, Z. R. Zileeva, J. V. Vakhitova, and V. V. Zarubaev, Nat. Prod. Res., https://www.tandfonline.com/doi/full/10.1080/14786419.2019.1696791, 2019.
K. Yu. Suponitskii, I. P. Tsypysheva, and A. V. Koval′skaya, J. Struct. Chem., 56, 188 (2015).
G. Luputiu and L. Gilau, Arch. Pharm., 302, 943 (1969).
I. V. Kulakov, Zh. M. Zhambekov, A. A. Ainabaev, S. D. Fazylov, and O. A. Nurkenov, Izv. Nats. Akad. Nauk Resp. Kaz., Ser. Khim., 4, 37 (2008).
Yu. V. Vakhitova, I. P. Tsypysheva, M. Kh. Salimgareeva, A. V. Kovalskaya, A. N. Lobov, U. Sh. Fatkullina, L. F. Zainullina, and M. S. Yunusov, Chem. Nat. Compd., 50, 498 (2014).
P. R. Petrova, A. V. Koval′skaya, A. N. Lobov, and I. P. Tsypysheva, Chem. Nat. Compd., 55, 1110 (2019).
P. R. Petrova, A. V. Koval′skaya, A. N. Lobov, and I. P. Tsypysheva, Chem. Nat. Compd., 55, 908 (2019).
I. P. Tsypysheva, A. V. Koval′skaya, I. U. Khalilova, Yu. Yu. Bakhtina, R. Yu. Khisamutdinova, S. F. Gabdrakhmanova, A. N. Lobov, F. S. Zarudii, and M. S. Yunusov, Chem. Nat. Compd., 50, 333 (2014).
J. Buckingham, K. H. Baggaley, A. D. Roberts, and L. F. Szabo, Dictionary of Alkaloids, 2nd Ed., CRC Press, 2010, 2374 pp.
Acknowledgment
The work was sponsored by a State Task for UfIC, UFRC, RAS, Topic No. AAAA-A20-120012090026-9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2020, pp. 763–766.
Rights and permissions
About this article
Cite this article
Petrova, P.R., Koval′skaya, A.V., Tsypysheva, I.P. et al. Synthesis of Several Cytisine Derivatives and their Cytotoxicities against A431, A375, and HCT 116 Tumor Cell Lines. Chem Nat Compd 56, 892–895 (2020). https://doi.org/10.1007/s10600-020-03177-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03177-x