Abstract
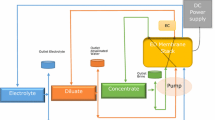
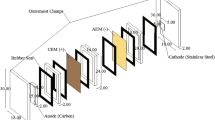
A technology for removing toxic Cd2+ from electroplating process electrolytes and washwater containing \({\text{SO}}_{4}^{{2 - }}\) or Cl– is proposed. This technology makes it possible to decrease the concentration of Cd2+ in treated solutions to 0.1–0.07 g/L and the Cd2+ ingress into plant waste waters by 288–366 times and attain a Cd2+ recovery of 99.7%. Electrolysis in a dual-chamber cell with an anion-exchange membrane makes it possible to return Cd and a H2SO4 solution back into the process. The removal of Cd2+ from Cl– containing solutions is recommended to be performed by membrane electrolysis, separating the insoluble anode with a chemically stable cation-exchange membrane to prevent the migration of Cl– from the catholyte into the anolyte and, correspondingly, their oxidation on the insoluble anode with the liberation of toxic Cl2.




Similar content being viewed by others
REFERENCES
Vinogradov, S.S., Ekologicheski bezopasnoe gal’vanicheskoe proizvodstvo (Environmentally Safe Galvanic Production), Moscow: Globus, 2002.
Perelygin, Yu.P., Flyagin, A.A., Zueva, T.V., and Zor’kina, O.V., Reagent technology for recycling highly concentrated solutions containing zinc, cadmium, copper, and ammonium ions, Reg. Arkhit. Stroit., 2009, no. 1, p. 87.
Smirnova, N.N. and Makarova, A.A., Efficiency of the use of chemical precipitation in the purification of wastewater generated from industrial electroplating processes from copper(II), zinc(II), nickel(II), and cadmium(II) ions, Voda: Khim. Ekol., 2018, nos. 1–3 (114), p. 134.
Alguacil, F.J. and Navarro, P., Permeation of cadmium through a supported liquid membrane impregnated with CYANEX 923, Hydrometallurgy, 2001, vol. 61, no. 2, pp. 137–142. https://doi.org/10.1016/S0304-386X(01)00163-3
Kozłowski, C., Apostoluk, W., Walkowiak, W., and Kita, A., Removal of Cr(VI), Zn(II) and Cd(II) ions by transport across polymer inclusion membranes with basic ion carriers, Physicochem. Probl. Miner. Process., 2002, vol. 36, no. 1, pp. 115–122.
Davydov, A.N. and Plokhov, S.V., Ion-exchange and electrochemical recovery of Cd(II) from rinsing water after galvanic metallization, Izv. Vyssh. Uchebn. Zaved.,Khim. Khim. Tekhnol., 2010, vol. 53, no. 2, pp. 129–130.
Elsherief, A.E., Removal of cadmium from simulated wastewaters by electrodeposition on spiral wound steel electrode, Electrochim. Acta, 2003, vol. 48, no. 18, pp. 2667–2673. https://doi.org/10.1016/S0013-4686(03)00314-1
Marder, L., Bernardes, A.M., and Ferreira, J.Z., Cadmium electroplating wastewater treatment using a laboratory-scale electrodialysis system, Sep. Purif. Technol., 2004, vol. 37, no. 3, pp. 247–255. https://doi.org/10.1016/j.seppur.2003.10.011
Dermentzis, K., Christoforidis, A., Papadopoulou, D., and Davidis, A., Ion and ionic current sinks for electrodeionization of simulated cadmium plating rinse waters, Environ. Prog. Sustainable Energy, 2011, vol. 30, no. 1, pp. 37–43. https://doi.org/10.1002/ep.10438
Shachneva, E.Yu., Alykov, N.M., and Archibasova, D.E., Adsorption of cadmium from aqueous solutions on modified sorbents, Tekh. Tekhnol. Pishch. Proizvod., 2012, no. 4, p. 171.
Yustratov, V.P. and Solov’eva, Yu.V., Development of adsorption technology for the purification of wastewater generated from industrial electroplating processes from heavy metal ions, Vestn. Kuzbasskogo Gos. Tekh. Univ., 2006, no. 1, p. 114.
Sanzhanova, S.S. and Zonkhoeva, E.L., Purification of mine drainage using natural sorbents, Voda: Khim. Ekol., 2016, no. 6 (96), p. 58.
Abonosimov, O.A., Lazarev, S.I., Strel’nikov, A.E., Dmitriev, V.M., and Kotenev, S.I., Ecological efficiency of electrobaromembrane removal of heavy metal ions from wastewater generated from agroindustrial enterprises, Nauka Tsentr. Ross., 2017, no. 5 (29), p. 101.
Turaev, D.Yu. and Kruglikov, S.S., Removal of cadmium ions from rinsing water in a recovery tank after cadmium plating in a sulfuric acid electrolyte, in Uspekhi v khimii i khimicheskoi tekhnologii (Advances in Chemistry and Chemical Engineering), Moscow: Ross. Khim.-Tekhnol. Univ. im. D.I. Mendeleeva, 2001, vol. 15, part 5, p. 59.
Turaev, D.Yu., Sirotkin, V.I., and Kruglikov, S.S., Removal of cadmium ions from rinsing water after cadmium plating: 1. An ammonium chloride electrolyte, Gal’vanotekh. Obrab. Poverkhn., 2001, vol. 9, no. 2, p. 45.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Turaev, D.Y., Kolesnikov, V.A. & Popov, N.A. Technology for the Purification of Electroplating Washwater from Cadmium Ions by Membrane and Membraneless Electrolysis. Theor Found Chem Eng 54, 200–207 (2020). https://doi.org/10.1134/S0040579520010224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520010224