Abstract
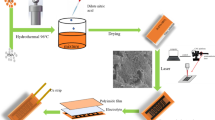
In this study, electrochemical hydrogen storage properties of graphene coatings were investigated. X-ray diffraction, Raman spectroscopy, field emission scanning electron microscopy, and galvanostatic charge/discharge measurements were used to characterize the materials properties. Graphene oxide was prepared using a modified Hummers method. A graphene coating was fabricated by electrophoretic deposition. Results showed that a graphene-coated copper electrode, as a low-cost and low-weight electrode, has a good charge/discharge capacity that makes it a promising candidate for electrochemical hydrogen storage applications. The highest discharge capacity obtained for a coated electrode was 45 mA h g–1, while the discharge capacity of a bare copper electrode was only 2 mA h g–1. A high capacity of the graphene coating is due to defects of the graphene structure such as wrinkles, crumples, etc., and oxygen-functional groups attached to graphene during syntheses process.







Similar content being viewed by others
REFERENCES
Amirante, R., Cassone, E., Distaso, E., and Tamburrano, P., Energy Convers. Manage., 2017, vol. 132, pp. 372–387.
Abdalla, A.M., Hossain, S., Nisfindy, O.B., Azad, A.T., et al., Energy Convers. Manage., 2018, vol. 165, pp. 602–627.
Kaur, M. and Pal, K., Int. J. Hydrogen Energy, 2016, vol. 41, no. 47, pp. 21861–21869.
Zhang, Y.H., Jia, Z.C., Yuan, Z.M., Yang, T., et al., J. Iron Steel Res. Int., 2015, vol. 22, no. 9, pp. 757–770.
Eftekhari, A. and Fang, B., Int. J. Hydrogen Energy, 2017, vol. 42, no. 40, pp. 25143–25165.
Yu, Y., Zhao, N., Shi, C., He, C., et al., Int. J. Hydrogen Energy, 2012, vol. 37, no. 7, pp. 5762–5768.
Wang, Y., Deng, W., Liu, X., and Wang, X., Int. J. Hydrogen Energy, 2009, vol. 34, no. 3, pp. 1437–1443.
Shiraz, H.G. and Shiraz, M.G., Int. J. Hydrogen Energy, 2017, vol. 42, no. 16, pp. 11528–11533.
Guo, G.F., Huang, H., Xue, F.H., Liu, C.J., et al., Surf. Coat. Technol., 2013, vol. 228, suppl. 1, pp. S120–S125.
Feng, H., Wei, Y., Shao, C., Lai, Y., et al., Int. J. Hydrogen Energy, 2007, vol. 32, no. 9, pp. 1294–1298.
Wu, M.S., Hsu, H.L., Chiu, H.H., and Lin, Y.P., Int. J. Hydrogen Energy, 2010, vol. 35, no. 17, pp. 8993–9001.
Bordbar, M., Alimohammadi, T., Khoshnevisan, B., Khodadadi, B., et al., Int. J. Hydrogen Energy, 2015, vol. 40, no. 31, pp. 9613–9620.
Mohammadi, M., Khoshnevisan, B., and Varshoy, Sh., Int. J. Hydrogen Energy, 2016, vol. 41, no. 24, pp. 10311–10315.
Shiraz, H.G. and Tavakoli, O., Renewable Sustainable Energy Rev., 2017, vol. 74, pp. 104–109.
Rajaura, R.S., Srivastava, S., Sharma, V., Sharma, P.K., et al., Int. J. Hydrogen Energy, 2016, vol. 41, no. 22, pp. 9454–9461.
Esfahani, S.L., Rouhani, S., and Ranjbar, Z., Surf. Interfaces, 2017, vol. 9, pp. 218–227.
Akhavan, O., Carbon, 2010, vol. 48, no. 2, pp. 509–519.
Pei, S. and Cheng, H.M., Carbon, 2012, vol. 50, no. 9, pp. 3210–3228.
Choi, M.H., Min, Y.J., Gwak, G.H., Paek, S.M., et al., J. Alloy Compd., 2014, vol. 610, pp. 231–235.
Tuinstra, F. and Koenig, J.L., J. Chem. Phys., 1970, vol. 53, no. 3, pp. 1126–1130.
Reich, S. and Thomsen, C., Philos. Trans. R. Soc., A, 2004, vol. 362, no. 1824, pp. 2271–2288.
Ferrari, A.C., Meyer, J.C., Scardaci, V., Casiraghi, C., et al., Phys. Rev. Lett., 2006, vol. 97, no. 18, p. 187401.
Qu, D., J. Power Sources, 2008, vol. 179, no. 1, pp. 310–316.
McCann, E., Phys. Rev. B, 2006, vol. 74, no. 16, p. 161403.
Akbarzadeh, R. and Dehghani, H., J. Solid State Electrochem., 2018, vol. 22, no. 2, pp. 395–405.
Min, Y.J., Lee, W.J., Gwak, G.H., Paek, S.M., et al., Bull. Korean Chem. Soc., 2015, vol. 36, pp. 2627–2631.
Chen, Y., Wang, Q., Zhu, Ch., Gao, P., et al., J. Mater. Chem., 2012, vol. 22, pp. 5924–5927.
Bleda-Martínez, M.J., Pérez, J.M., Linares-Solano, A., Morallón, E., et al., Carbon, 2008, vol. 46, pp. 1053–1059.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Reza Ghorbani, Sahand Behrangi Electrochemical Hydrogen Storage Properties of Graphene Coating Formed by Electrophoretic Deposition. Surf. Engin. Appl.Electrochem. 56, 22–27 (2020). https://doi.org/10.3103/S1068375520010056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375520010056