Abstract
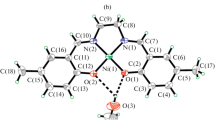
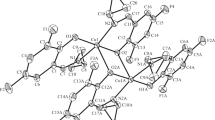
Reactions of Cr, Mo, W, and Fe metal carbonyls with potentially heptadentate (S3N4) tripodal Schiff base ligand, (thio)3tren, have been studied. The Schiff base (thio)3tren has been prepared by condensation of thiophene-2-carboxaldehyde with tris(2-aminoethyl)amine. The Schiff base and its metal complexes have been characterized by IR, UV-Vis, 1H and 13C NMR spectra, TGA, and elemental analysis. According to the spectroscopic data, (thio)3tren is coordinated to the metal centers as a potentially heptadentate ligand. The products have been tested in vitro against the bacterial species, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus cereus by the disc diffusion and micro-broth dilution methods, and characterized by antibacterial activity higher than that of the ligand. The lowest MIC was determined for Fe complex against Bacillus cereus and the highest for Mo complex against Escherichia coli.


Similar content being viewed by others
REFERENCES
Kirtley, S.W., Wilkinson, G., Gordon, F., Stone, A., and Abel, E.W., Comprehensive Organometallic Chemistry, vol. 3, Oxford: Pergamon Press, 1982, p. 783.
Collman, J.P. and Hegedus, L.S., Principles and Application of Organotransition Metal Chemistry, California: University Science Book, 1980
Waern, J.B. and Harding M.M., J. Organomet. Chem., 2004, vol. 689, p. 4655. https://doi.org/10.1016/j.jorganchem.2004.08.014
Stohs, S.J. and Bagchi, D., Free Radic. Biol. Med., 1995, vol. 18, p. 321. https://doi.org/10.1016/0891-5849(94)00159-h
Taylor, S., Kammerer, B., and Bayer, E., Chem. Rev., 1997, vol. 97, p. 333. https://doi.org/10.1021/cr940467q
Jelikić-Stankov, M., Uskoković-Markovic, S., Holclajtner-Antunovic, I., Todorovic, M., and Djurdjevic, P., J. Trace Elem. Med. Bio., 2007, vol. 21, p. 8. https://doi.org/10.1016/j.jtemb.2006.11.004
Soliman, A.A. and Mohamed, G.G., Thermochim. Acta, 2004, vol. 421, p. 151. https://doi.org/10.1016/j.tca.2004.03.010
Piper J.M. and Lovell, S.J., Anal. Biochem., 1981, vol. 117. p. 70. https://doi.org/10.1016/0003-2697(81)90693-x
Jelikić-Stankov, M. and Veselinović, D., Analyst, 1989, vol. 114, p. 719. https://doi.org/10.1039/AN9891400719
Jelikić-Stankov, M., Veselinović, D., Malesěv, D., and Radović, Z., J. Pharm. Biomed. Anal., 1989, vol. 7, p. 1565. https://doi.org/10.1016/0731-7085(89)80166-9
Jelikić-Stankov, M., Malesěv, D., Veselinović, D., and Radovic, Z., Polyhedron, 1991, vol. 10, p. 455. https://doi.org/10.1016/S0277-5387(00)80211-9
Wang, M., Wang, L.F., Li, Y.Z., Li, Q.X., Xu, Z.D., and Qu, D.Q., Transition Met. Chem., 2001, vol. 26, p. 307. https://doi.org/10.1023/A:10071593
Yamase, T., Mol. Eng., 1993, vol. 3, p. 241. https://doi.org/10.1007/BF00999636
Dhumwad, S.D., Gudasi, K.B., and Goudar, T.R., Indian J. Chem. A, 1994, vol. 33, p. 320.
Reddy, K.H., Reddy, P.S., and Babu, P.R., Transition Met. Chem., 2000, vol. 25, p. 154. https://doi.org/10.1023/A:1007027011216
Tarasconi, P., Capacchi, S., Pelosi, G., Corina, M., Albertini, R., Bonati, A., Dall’Aglio, P.P., Lunghi, P., and Pinelli, S., Bioorg. Med. Chem., 2000, vol. 8, p. 157. https://doi.org/10.1016/S0968-0896(99)00260-6
Charo, J., Lindencrona, J.A., Carlson, L.M., Hinkula, J., and Kiessling, R., J. Virol., 2004, vol 78, p. 11321. https://doi.org/10.1128/JVI.78.20.11321-11326.2004
West, D.X., Dietrich, S.L., Thientananvanich, I., Brown, C.A., and Liberta, A.E., Transition Met. Chem., 1994, vol. 19, p. 195. https://doi.org/10.1007/BF00161888
Mioc, U., Todorovic, M., Davidovic, M., Colomban, P., and Holclajtner-Antunovic I., Solid State Ionics, 2005, vol. 176, p. 3005. https://doi.org/10.1016/j.ssi.2005.09.056
Das, A. Bajaj, H.C., Venkatasubramanian, K., and Bhadbhade, M.M., Polyhedron, 1995, vol. 14, p. 495. https://doi.org/10.1016/0277-5387(94)00274-I
Kay, D.L. Daumann, L.J., Hanson, G.R., and Gahan, L.R., Polyhedron, 2013, vol. 64, p. 151. https://doi.org/10.1016/j.poly.2013.03.030
Pillai, M.R.A., Samuel, G., Banerjee, S., Mathew, B., Sarma, H.D., and Jurisson, S., Nucl. Med. Biol., 1999, vol. 26, p. 69. https://doi.org/10.1016/s0969-8051(98)00051-1
Percy, G.C. and Thornton, J., J. Inorg. Nucl. Chem., 1973, vol. 35, p. 2319. https://doi.org/10.1016/0022-1902(73)80296-9
Tweedy, B.G., Phytopathology, 1964, vol. 55, p. 910.
Parekh, J., Inamdhar, P., Nair, R., Baluja, S., and Chanda, S., J. Serb. Chem. Soc., 2005, vol. 70, p. 1161. https://doi.org/10.2298/JSC0510155P
Alyea, E.C., Liu, Sh., Li, B., Xu, Z., and You, X., Acta Cryst. C, 1989, vol. 45, p. 1566. https://doi.org/10.1107/S0108270189002283
Matin, S.J. and Khojasteh, R.R., Russ. J. Gen. Chem., 2015, vol. 85, p. 1763. https://doi.org/10.1134/S1070363215070312
Ranjineh Khojasteh, R. and Jalali Matin, S., Russ. J. App. Chem., 2015, vol. 88, p. 921. https://doi.org/10.1134/S107042721506004X
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Rahmatabadi, F.D., Khojasteh, R.R., Fard, H.K. et al. New Cr, Mo, W, and Fe Metal Complexes with Potentially Heptadentate (S3N4) Tripodal Schiff Base Ligand: Synthesis, Characterization, and Antibacterial Activity. Russ J Gen Chem 90, 1317–1321 (2020). https://doi.org/10.1134/S1070363220070191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220070191