Abstract
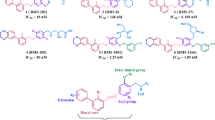
The high expression of the translocator protein (TSPO) makes it an ideal target for imaging and therapy. The present study is aimed at optimizing acetamidobenzoxazolone-based TSPO ligands by structural modification to overcome the limitations of TSPO ligands of the first two generations such as nonspecificity and polymorphism. Three different TSPO proteins, 2MGY, 4RYQ, and 4UC1, in the native and mutated form were chosen. Acetamidobenzoxazolones modified with phenylalanine methyl ester (methyl 2-(2-(5-bromo/chloro-2-oxobenzooxazol-3(2H)-yl)acetamido)-3-phenylpropanoate, ABPO-Br, ABPO-Cl) through better or comparable docking scores than the known TSPO ligands such as MBMP, FEBMP, FPBMP, and PK11195 are identified as potential TSPO ligands. ABPO-Cl and ABPO-Br were synthesized using phenylalanine methyl ester as a moiety for incorporation of the desired pharmacophoric feature. All the intermediates and final compounds were purified using column chromatography and analytical HPLC (purity > 97%). The purified compounds were characterized by 1H, 13C NMR and mass spectroscopy. Drug likeliness and comparative bioactivity analysis were performed using QikProp through prediction of various properties. Both analogs follow all the five Lipinski rules and three Jogersen’s rules predicting their drug likeliness. The other important aspects related to TSPO ligands such as blood-brain barrier penetration and better contrast have been predicted through lipophilicity (QP log P = 2.76 and 2.74 for ABPO-Br and ABPO-Cl, respectively) and serum binding (QP log Khsa = −0.18 and −0.25 for ABPO-Br and ABPO-Cl, respectively). The selectivity and distribution of these TSPO ligands were confirmed by 99mTc-ABPO-Br dynamic image in New Zealand rabbit. These results have shown that the ABPO analogs have the potential to act as better ligands as compared to known acetamidobenzoxazolone derivatives and would be of interest as a promising starting point for designing compounds for TSPO targeting.
Similar content being viewed by others
References
Papadopoulos, V., Baraldi, M., Guilarte, T.R., Knudsen, T.B., Lacapère, J.J., Lindemann, P., Norenberg, M.D., Nutt, D., Weizman, A., Zhang, M.R., and Gavish, M., Trends Pharmacol. Sci., 2006, vol. 27, pp. 402–409.
Li, F., Liu, J., Liu, N., Kuhn, L.A., Garavito, R.M., and Miller, S.F., Biochemistry, 2016, vol. 55, pp. 2821–2831.
Li, F., Liu, J., Garavito, R.M., and Miller, S.F., Pharmacol. Res., 2015, vol. 99, pp. 404–409.
Albrecht, D.S., Granziera, C., Hooker, J.M., and Loggia, M.L., ACS Chem. Neurosci., 2016, vol. 7, pp. 470–483.
Kreisl, W.C., Fujita, M., Fujimura, Y., Kimura, N., Jenko, K.J., Kannan, P., Hong, J., Morse, C.L., Zoghbi, S.S., Gladding, R.L., Jacobson, S., Oh, U., Pike, V.W., and Innis, R.B., Neuroimage, 2010, vol. 49, pp. 2924–2932.
Denora, N., Iacobazzi, R.M., Natile, G., and Margotta, N., Coord. Chem. Rev., 2017, vol. 341, pp. 1–18.
Denora, N., Margiotta, N., Laquintana, V., Lopedota, A., Cutrignelli, A., Losacco, M., Franco, M., and Natile, G., ACS Med. Chem. Lett., 2014, vol. 5, pp. 685–689.
Milite, C., Barresi, E., Pozzo, E.D., Costa, B., Viviano, M., Porta, A., Messere, A., Sbardella, G., Settimo, F.D., Novellino, E., Cosconati, S., Castellano, S., Taliani, S., and Martini, C., J. Med. Chem., 2017, vol. 60, no. 18, pp. 7897–7909. https://doi.org/10.1021/acs.jmedchem.7b01031
Zhang, M.R., Kida, T., Noguchi, J., Furutsuka, K., Maeda, J., Suhara, T., and Suzuki, K., Nucl. Med. Biol., 2003, vol. 30, pp. 513–519.
James, M.L., Fulton, R.R., Vercoullie, J., Henderson, D.J., Garreau, L., Chalon, S., Dollé, F., Selleri, S., Guilloteau, D., and Kassiou, M., J. Nucl. Med., 2008, vol. 49, pp. 814–822.
Briard, E., Zoghbi, S.S., Imaizumi, M., Gourley, J.P., Shetty, H.U., Hong, J., Cropley, V., Fujita, M., Innis, R.B., and Pike, V.W., J. Med. Chem., 2008, vol. 51, pp. 17–30.
Zhang, M.R., Kumata, K., Maeda, J., Yanamoto, K., Hatori, A., Okada, M., Higuchi, M., Obayashi, S., Suhara, T., and Suzuki, K., J. Nucl. Med., 2007, vol. 48, pp. 1853–1861.
Owen, D.R., Yeo, A.J., and Gunn, R.N., J. Cereb. Blood Flow Metab., 2012, vol. 32, pp. 1–5.
Fukaya, T., Kodo, T., Ishiyama, T., Kakuyama, H., Nishikawa, H., Baba, S., and Masumoto, S., Bioorg. Med. Chem., 2012, vol. 22, pp. 5568–5582.
Fukaya, T., Ishiyama, T., Baba, S., and Matsumoto, S., J. Med. Chem., 2013, vol. 56, pp. 8191–8195.
Tiwari, A.K., Yui, J., Fujinaga, M., Kumata, K., Shimoda, Y., Yamasaki, T., Xie, L., Hatori, A., Maeda, J., Nengaki, N., and Zhang, M.R., J. Neurochem., 2014, vol. 129, pp. 712–720.
Tiwari, A.K., Fujinaga, M., Yui, J., Yamasaki, T., Xie, L., Kumata, K., Mishra, A.K., Shimoda, Y., Hatori, A., Ji, B., Ogawa, M., Kawamura, K., Wang, F., and Zhang, M.R., Org. Biomol.Chem., 2014, vol. 12, pp. 9621–9630.
Tiwari, A.K., Ji, B., Fujinaga, M., Yamasaki, T., Xie, L., Luo, R., Shimoda, Y., Kumata, K., Zhang, Y., Hatori, A., Maeda, J., Higuchi, M., Wang, F., and Zhang, M.R., Theranostics, 2015, vol. 5, pp. 961–969.
Tiwari, A.K., Yui, J., Zhang, Y., Fujinaga, M., Yamasaki, T., Xie, L., Shimoda, Y., Kumata, K., Hatori, A., and Zhang, M.R., RSC Adv., 2015, vol. 5, no. 123, pp. 101447–101454.
Kumari, N., Chadha, N., Srivastava, P., Mishra, L.C., Bhagat, S., Mishra, A.K., and Tiwari, A.K., Chem. Biol. Drug. Des., 2017, vol. 90, no. 4, pp. 511–519.
Fujinaga, M., Luo, R., Kumata, K., Zhang, Y., Hatori, A., Yamasaki, T., Xie, L., Mori, W., Kurihara, Y., Ogawa, M., Nengaki, N., Wang, F., and Zhang, M.R., J. Med. Chem., 2017, vol. 60, pp. 4047–4061.
Srivastava, P., Kaul, A., Ojha, H., Kumar, P., and Tiwari, A.K., RSC Adv., 2016, vol. 6, no. 115, pp. 114491–114499.
Hanaoka, H., Ohshima, Y., Suzuki, Y., Yamaguchi, A., Watanabe, S., Uehara, T., Nagamori, S., Kanai, Y., Ishioka, N.S., Tsushima, Y., Endo, K., and Arano, Y., J. Nucl. Med., 2015, vol. 56, pp. 791–797.
Kang, F.N., SpringerPlus, 2013, vol. 2, article no. 353.
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug. Deliv. Rev., 1997, vol. 23, pp. 3–25.
Lipinski, C.A., J. Pharmacol. Toxicol. Meth., 2000, vol. 44, pp. 235–249.
Jorgensen, W.L. and Duffy, E.M., Adv. Drug Deliv. Rev., 2002, vol. 54, pp. 355–366.
Jorgensen, W.L. and Duffy, E.M., Bioorg. Med. Chem. Lett., 2000, vol. 10, pp. 1155–1158.
Veber, D.F., Johnson, S.R., Cheng, H.Y., Smith, B.R., Ward, K.W., and Kopple, K.D., J. Med. Chem., 2002, vol. 45, pp. 2615–2623.
Irwin, J.J. and Shoichet, B.K., J. Med. Chem., 2016, vol. 59, pp. 4103–4120.
Pagadala, N.S., Syed, K., and Tuszynski, J., Biophys. Rev., 2017, vol. 9, pp. 91–102.
Chaput, L. and Mouawad, L., J. Cheminform., 2017, vol. 9, p. 37.
Bhargavi, M., Sivan, S.K., and Potlapally, S.R., Comp. Biol. Chem., 2017, vol. 68, pp. 43–55.
Kakkar, D., Tiwari, A.K., Chuttani, K., Kaul, A., Singh, H., and Mishra, A.K., Cancer Biotherapy and Radiopharmaceuticals, 2010, vol. 25(6), pp. 645–655.
Guo, Y., Kalathur, R.C., Liu, Q., Kloss, B., Bruni, R., Ginter, C., Kloppmann, E., Rost, B.C., and Hendrickson, W.A., Science, 2015, vol. 347, pp. 551–555.
Li, F., Liu, J., Zheng, Y., Garavito, R.M., and Miller, S.F., Science, 2015, vol. 347, pp. 555–558.
Tanwar, J., Datta, A., Tiwari, A.K., Thirumal, M., Chuttani, K., and Mishra, A.K., Bioconjug Chem., 2011, vol. 22(2), pp. 244–255.
Srivastava, P., Tiwari, A.K., Chadha, N., Chuttani, K., and Mishra, A.K., Eur. J. Med. Chem., 2013, vol. 65, pp. 12–20.
Sinha, D., Shukla, G., Tiwari, A.K., Chaturvedi, S., Chuttani, K., Chandra, H., and Mishra, A.K., Chem. Biol. Drug. Des., 2009, vol. 74, pp. 159–164.
Singh, S., Tiwari, A.K., Varshney, R., Mathur, R., Hazari, P.P., Singh, B., and Mishra, A.K., RSC Adv., 2015, vol. 5, pp. 41977–41984.
Acknowledgments
The authors are grateful to Dr. Tarun Sekhri, Director of INMAS, Dehli, Dr. A. K. Mishra, Head of the Division of Cyclotron and Radiopharmaceutical Science of INMAS, and Dr. Gaurav Mittal, Head of CCM for providing the facility to carry out the experiments. The authors are also grateful to Dr. Himanshu Ojha, Scientist, Dr. Sonia Gandhi, Scientist, Mr. Sunil Pal, Technical Officer, and Mr. Harish Rawat, INMAS, for their technical support.
Funding
The study was supported by INMAS, Delhi, under Task Project ST/15-16/INM-03.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Srivastava, P., Kumar, P. & Tiwari, A.K. Design, Synthesis, and In Silico Evaluation of Methyl 2-(2-(5-Bromo/chloro-2-oxobenzoxazol-3(2H)-yl)-acetamido)-3-phenylpropanoate for TSPO Targeting. Radiochemistry 62, 107–118 (2020). https://doi.org/10.1134/S1066362220010142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362220010142