Abstract—
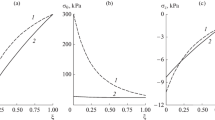
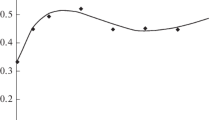
The plane problem of the effect of contractions of smooth muscle cells in the wall of a resistance vessel under the action of transmural pressure on the radius and the distribution of stresses in the vascular wall is considered. It is assumed that in the inactivated state the vessel wall is hyperelastic, and the contraction of smooth muscle cells as a result of activation contributes to the circumferential stress alone. Based on the model and published experimental data, a functional dependence of the active stress on the concentration of the activator of smooth muscle contraction is obtained. Calculations show that the total stress in the wall is determined mainly by the active component. With increasing pressure, contractions of smooth muscle cells lead to decrease in stresses, while the pattern of the distribution of circumferential stresses changes. The circumferential stretch ratios also decrease with activation and their distribution becomes more homogeneous. In both passive and active vessels, the modulus of the ratio of the radial to circumferential stress decreases with increase in tension, this ratio being several times greater in the active vessel than in the passive one.





Similar content being viewed by others
REFERENCES
M. J. Davis, “Perspective: physiological role(s) of the vascular myogenic response,” Microcirculation 19, 99—114 (2012). https://doi.org/10.1111/j.1549-8719.2011.00131.x
B. E. Carlson and D. A. Beard, “Mechanical control of cation channels in the myogenic response,” Am. J. Physiol. Heart Circ. Physiol. 301 (2), H331–H343 (2011). https://doi.org/10.1152/ajpheart.00131.2011
H. J. Knot and M.T. Nelson, “Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure,’’ J. Physiol. 508 (1), 199–209 (1998).
H. Chen and G. S. Kassab, ”Microstructure-based biomechanics of coronary arteries in health and disease,” J. Biomech 49, 2548–2559 (2016). https://doi.org/10.1016/j.jbiomech.2016.03.023
G. A. Holzapfel and R. W. Ogden, “Constitutive modelling of arteries. Review,” Proc. R. Soc. A, 466, 1551–1597 (2010). https://doi.org/10.1098/rspa.2010.0058
B. E. Carlson, J. C. Arciero, and T. W. Secomb, “Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses,” Amer. J. Phys. Heart Circ. Phys. 295, H1572–H1579 (2008). https://doi.org/10.1152/ajpheart.00262.2008
J. D. Humphrey and S. Na, “Elastodynamics and arterial wall stress,” Ann. Biomed. Eng. 30, 509–523 (2002). https://doi.org/10.1114/1.1467676
N. Kleinstreuer, T. David, M. J. Plank, and Z. Endre, “Dynamic myogenic autoregulation in the rat kidney: a whole-organ model,” American J. Phys. Renal Physiol. 294, F1453–F1464 (2008). https://doi.org/10.1152/ajprenal.00426.2007
E. VanBavel and B. G. Tuna, “Integrative modeling of small artery structure and function uncovers critical parameters for diameter regulation,” PLoS ONE 9 (1), e86901 (2014). https://doi.org/10.1371/journal.pone.0086901
S. Baek, R. L. Gleason, K. R. Rajagopa, and J. D. Humphrey, “Theory of small on large: potential utility in computations of fluid–solid interactions in arteries,” Comput. Meth. Appl. Mech. Eng. 196, 3070–3078 (2007). https://doi.org/10.1016/j.cma.2006.06.018
H. Chen and G. S. Kassab, “Microstructure-based constitutive model of coronary artery with active smooth muscle contraction,” Scientific Reports 7 (1), 9339 (2017). https://doi.org/10.1038/s41598-017-08748-7
Y. Huo, Y. Cheng, X. Zhao, et al., “Biaxial vasoactivity of porcine coronary artery,” Am. J. Physiol. Heart Circ. Physiol. 302 (10), H2058–H2053 (2012). https://doi.org/10.1152/ajpheart.00758.2011
Y. Huo, X. Zhao, Y. Cheng, et al., “Two-layer model of coronary artery vasoactivity,” J. Appl. Physiol. 114 (10), 1451–1459 (2013). https://doi.org/10.1152/japplphysiol.01237.2012
Y. Lu, H. Wu, J. Li, et al., “Passive and active triaxial wall mechanics in two-layer model of porcine coronary artery,” Scientific Reports 7 (1), 13911 (2017). https://doi.org/10.1038/s41598-017-14276-1
B. Zhou, A. Rachev, and T. Shazly, “The biaxial active mechanical properties of the porcine primary renal artery,” J. Mech. Behav. Biomed. Mater. 48, 28–37 (2015). https://doi.org/10.1016/j.jmbbm.2015.04.004
C. G. Caro,T. J. Pedley, R. C. Schroter, and W. A. Seed, The Mechanics of the Circulation (Oxford University Press, 1978).
Physiology of Blood Circulation: Physiology of the Vascular System (Nauka, Leningrad, 1984) [in Russian].
G. L. Baumbach, J. G. Walmsley, and M. N. Hart, “Composition and mechanics of cerebral arterioles in hypertensive rats,” Am. J. Pathol. 133 (3), 464–471 (1988).
A. E. Green and J. E. Adkins, Large Elastic Deformations and Non-Linear Continuum Mechanics (Clarendon Press, Oxford, 1960).
Y. Fung, Biomechanics (Springer, New-York, 1981).
E. D. Hogestätt, K.-E. Andersson, and L. Edvinsson, ``Mechanical properties of rat cerebral arteries as studied by a sensitive device for recording of mechanical activity in isolated small blood vessels,’’ Acta Physiol. Scand. 117, 49–61 (1983).
N. Kh. Shadrina, “The Lamé problem applied to a blood vessel with an active wall,” Biophysics 63 (4), 629–636 (2018).
S. Murtada, M. Kroon, and G. A. Holzapfel, “A calcium-driven mechanochemical model for prediction of force generation in smooth muscle,” Biomech. Model. Mechanobiol. 9, 749–762 (2010). https://doi.org/10.1007/s10237-010-0211-0
A. Arner, “Mechanical characteristics of chemically skinned guinea-pig Taenia coli,” Eur. J. Physiol. 395, 277–284 (1982).
R. Sanft, A. Power, and C. Nicholson, “Modeling the effects of muscle contraction on the mechanical response and circumferential stability of coronary arteries,” Mathem. Biosci. 315, 108223 (2019). https://doi.org./10.1016/j.mbs.2019.108223
M.A. Zulliger, A. Rachev, and N. Stergiopulos, “A constitutive formulation of arterial mechanics including vascular smooth muscle tone,” Am. J. Physiol. Heart Circ. Physiol. 287, H1335–H1343 (2004). https://doi.org/10.1152/ajpheart.00094.2004
Funding
The work was financially supported by the Program of Fundamental Scientific Research of State Academies for 2013–2020 (GP-14, Section 64).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Shadrina, N.K. Investigation of the Influence of Smooth Muscle Contractions on the Properties of the Wall of a Small Arterial Vessel. Fluid Dyn 55, 145–153 (2020). https://doi.org/10.1134/S001546282002010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S001546282002010X