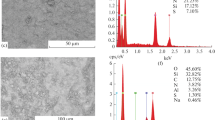
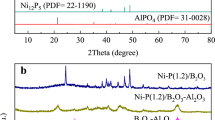
Abstract
A method for the surface chemical modification of aluminum oxyhydroxide (boehmite γ-AlO (OH)) by the nitrilotris(methylene phosphonic) acid (NTP) complexing ligand is proposed. The unmodified and NTP-modified boehmites are characterized using X-ray powder diffraction, XPS, and IR spectroscopy; the acid–base and complex-forming properties of surface-grafted NTP are studied. One of the three phosphonic groups of NTP is found to be involved in binding to the boehmite surface. The surface concentration and stepwise dissociation constants of grafted NTP are determined. A study of the nickel(II) sorption as a function of aqueous acidity shows that the modifying coating increases the sorption capacity of boehmite (causing рН50 to shift by one unit toward lower values). In terms of surface complexation theory, nickel(II) sorption from aqueous solutions may be described by models involving ≡Al–ONi+ and ≡Al–ONi(OH) complexes in the case of boehmite and ≡Al–LHi Nii–3 (i = 0, 1, 2, or 3) complexes in the case of NTP-modified boehmite (NTP-boehmite). NTP anchorage to the surface decreases the stability of nickel(II) complexes compared to their analogues in solutions. A mechanism of nickel(II) ion binding by NTP-boehmite is suggested. The prepared new organomineral support can be used to immobilize those metal ions that form stable complexes with phosphonic acids.






Similar content being viewed by others
REFERENCES
M. Manyangadze, N. H. M. Chikuruwo, T. B. Narsaiah, et al., S. Afr. J. Chem. Eng. 31, 25 (2020). https://doi.org/10.1016/j.sajce.2019.11.003
A. Y. Olenin and G. V. Lisichkin, Russ. J. Gen. Chem. 89, 1101 (2019). https://doi.org/10.1134/S1070363219070168
Chemistry of Grafted Surface Compounds, Ed. by G. V. Lisichkin (Fizmatlitgiz, Moscow, 2003) [in Russian].
P. G. Mihgalyov and G. V. Lisichkin, Russ. Chem. Rev. 75, 541 (2006). https://doi.org/10.1002/chin.200645245
C. Queffélec, M. Petit, P. Janvier, et al., Chem. Rev. 112, 3777 (2012). https://doi.org/10.1021/cr20042121
S. P. Pujari, L. Scheres, T. M. Marcelis, and H. Zuilhof, Angew. Chem., Int. Ed. Engl. 51, 6322 (2014). https://doi.org/org/10.1002/anie.201306709
G. V. Lisichkin and A. Y. Olenin, Russ. J. Appl. Chem. 93, 1 (2020). https://doi.org/10.31857/S0044461820010016
M. Mohapatra and P. Pramanic, Colloids and Surfaces: PhysicoChem. Eng. Aspects 339, 35 (2009). https://doi.org/10.1016/j.colsurfa.2009.01.009
J. Wang, Y. Liu, Z. Wang, P. Wang, et al., Int. J. Hydrogen En. 44, 16575 (2019). https://doi.org/10.1016/j.ijhydene.2019.04.192
B. Nowack and A. T. Stone, J. Colloid Interface Sci. 214, 20 (1999). https://doi.org/10.1006/jcis.1999.6111
Yu. V. Kholin, V. N. Zaitsev, G. N. Zaitseva, et al., Russ. J. Inorg. Chem. 62, 275 (1995).
M. Das, D. Mishra, P. Dhak, et al., Small 5, 2883 (2009). https://doi.org/10.1002/smll.200901219
M. Zenobi and E.H. Rueda, Quim. Nova 35, 505 (2012). https://doi.org/10.1590/s0100-40422012000300012
T. N. Kropacheva, A. S. Antonova, and V. I. Kornev, Mendeleev Commun. 29, 358 (2019). https://doi.org/10.1016/j.mencom.2019.05.040
L. S. Kostenko, S. A. Akhmedov, and V. N. Zaitsev, Metody Ob”ekty Khim. Anal. 1, 116 (2006). https://doi.org/10.17721/moca
The Environmental Chemistry of Aluminum, Ed. by G. Sposito (CRC Press, 1996).
L. Rajabi and A. Derakhshan, Sci. Adv. Mater. 2, 163 (2010). https://doi.org/10.1166/sam.2010.1063
Stability Constants Computation Programs: Hyperquad 2008, Hyperquad Simulation and Speciation HySS2009. www.hyperquad.co.uk
PND F 14.1:46-9: Quantitative Chemical Analysis of Waters (Moscow, 1996) [in Russian].
R. Zhao, P. Rupper, and S. Gaan, Coatings 7, 133 (2017). https://doi.org/10.3390/coatings7090133
M. C. Zenobi, C. V. Luengo, M. J. Avena, and E. H. Rueda, Spectrochim. Acta A 75, 1283 (2010). https://doi.org/10.1016/j.ssa.2009.12.059
B. V. A. Rao, M. V. Rao, S. S. Rao, and B. Sreedhar, J. Surf. Eng. Mater. Adv. Technol. 3, 28 (2013). https://doi.org/10.4236/jsemat.2013.31005
S. H. Wang, C. S. Liu, F. J. Shan, and G. C. Qi, Acta Metall. Sin. (Engl. Lett.) 21, 355 (2008). https://doi.org/10.1016/s1006-7191(08)60059-9
T. N. Kropacheva, A. S. Antonova, and V. I. Kornev, Russ. J. Inorg. Chem. 62, 150 (2017). https://doi.org/10.1134/S0036023617020103
L. D. Pettit and H. K. J. Powell, IUPAC Stability Constants Database, Version 4.74 (Academic Software). www.acadsoft.co.uk.
V. Deluchat, J.-C. Bollinger, B. Serpaud, and C. Caullet, Talanta 44, 897 (1997). https://doi.org/10.1016/S0039-9140(96)02136-4
K. Sawada, T. Araki, T. Suzuki, and K. Doi, Inorg. Chem. 28, 2687 (1989). https://doi.org/10.1021/ic00312a036
N. V. Somov, F. F. Chausov, R. M. Zakirova, and I. V. Fedotova, Crystallogr. Rep. 61, 238 (2016). https://doi.org/10.1134/s1063774516020243
P. Pavlova and L. Sigg, Water Res. 22, 1571 (1988). https://doi.org/10.1016/0043-1354(88)90170-4
D. G. Kinniburgh, M. L. Jackson, and J. K. Syers, Soil Sci. Soc. Am. J. 40, 796 (1976). https://doi.org/10.2136/sssaj1976.03615995004000050047x
Surface Complexation Modelling, Ed. by J. Lutzenkirchen (Academic Press, 2006).
M. A. Islam, M. J. Angove, and D. W. Morton, J. Water Process Eng. 32, 100964 (2019). https://doi.org/10.1016/j.jwpe.2019.100964
L. B. Zubakova, A. S. Tevlina, and A. B. Davankov, Synthetic Ion-Exchange Materials (Khimiya, Moscow, 1978) [in Russian].
A. W. Trochimczuk and J. Jezierska, J. Inorg. Organomet. Polym. 10, 81 (2000). https://doi.org/10.1023/A:1009423925041
ACKNOWLEDGMENTS
The authors are thankful to R.M. Zakirova, Candidate of Science in physics and mathematics, Leading Engineer of the Laboratory of X-ray Structural Analysis, at Educational and Research Institute of Experimental Natural Sciences; Institute of Mathematics, Information Technology and Physics; Udmurt State University, for help with X-ray analysis.
Funding
The study used equipment of the shared facilities center at Udmurt State University and the Center for Physical and Physicochemical Methods of Analysis and Characterization of Surfaces, Nanostructures, Materials, and Articles of Manufacture at the Udmurt Federal Research Center, Ural Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Kropacheva, T.N., Gazizyanova, A.R. & Gil’mutdinov, F.Z. New Complex-Forming Organomineral Support Based on Aluminum Oxyhydroxide Modified with Nitrilotris(methylene phosphonic) Acid. Russ. J. Inorg. Chem. 65, 1150–1159 (2020). https://doi.org/10.1134/S0036023620080070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620080070