Abstract
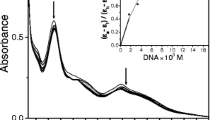
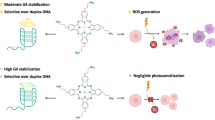
Two ruthenium complexes containing nitro-substituted ligand, [Ru(bpy)2(hnpip)](PF6)2 (1) and [Ru(phen)2(hnpip)](PF6)2 (2) (bpy = 2,2'-bipyridine; phen = 1,10-phenanthroline; hnpip = 2-(3-hydroxyl- 4-nitrophenyl)imidazo[4,5-f][1,10-phenanthroline] were synthesized and characterized. DNA binding behaviors were studied by luminescent titration, UV-vis titration, FRET (FRET = Fluorescence resonance energy transfer) and molecular docking experiments. Complex 2 exhibited “light switch” effect upon addition of G-quadruplex DNA and displayed fluorescent selectivity towards G-quadruplex DNA over other DNAs in the presence of K4[Fe(CN)6]. Fluorescence experiments, FRET experiments and molecular docking results indicated that the selectivity may be attributed to protection of nitro group from water, DNA affinity and the difference in DNA structures. Furthermore, both complexes were found to cleave DNA under irradiation by the formation of singlet oxygen. DNA topoisomerase inhibition experiments indicated that complex 2 exhibited higher topoisomerase I inhibition activity (IC50 = 18 μM) than complex 1 (IC50 = 50 μM). Docking studies revealed that complex 2 stabilized Top1cc complex via π–π interaction and the formation of hydrogen bond.









Similar content being viewed by others
REFERENCES
L. F. Liu, Ann. Rev. Biochem. 58, 351 (1989). https://doi.org/10.1146/annurev.bi.58.070189.002031
N. M. Baker, R. Rajan, and A. Mondragon, Nucleic Acids Res. 37, 693 (2009). https://doi.org/10.1093/nar/gkn1009
G. Capranico, J. Marinello, and G. Chillemi, J. Med. Chem. 60, 2169 (2017). https://doi.org/10.1021/acs.jmedchem.6b00966
M. Li and Y. Liu, Genom. Proteom. Bioinf. 14, 166(2016). https://doi.org/10.1016/j.gpb.2016.02.004
Y. Pommier, Y. Sun, S. N. Huang, et al., Nat. Rev. Mol. Cell Biol. 17, 703 (2016). https://doi.org/10.1038/nrm.2016.111
Y. Pommier, E. Leo, H. Zhang, et al., Chem. Biol. 17, 421 (2010). https://doi.org/10.1016/j.chembiol.2010.04.012
Y. Pommier, C.Marchand, Nat. Rev. Drug Discovery 11, 25 (2012). https://doi.org/10.1038/nrd3404
K. E. Hevener, T. A.Verstak, K. E. Lutat, et al., Acta Pharm. Sin. 8, 844 (2018). https://doi.org/10.1016/j.apsb.2018.07.008
S. M. Cuya, M. A. Bjornsti, and R. C. A. M. Waardenburg, Cancer Chemoth. Pharm. 80, 1 (2017). https://doi.org/10.1007/s00280-017-3334-5
V. Nagaraja, A.A. Godbole, and S. R. Henderson, Drug Discov. Today, 22, 510 (2017). https://doi.org/10.1016/j.drudis.2016.11.006
M. K. Kathiravan, A. N. Kale, and S. Nilewar, Mini-Rev. Med. Chem. 16, 1219 (2016). https://doi.org/10.2174/1389557516666160822110819
K. Gokduman, Curr. Drug Targets 16, 1928 (2016). https://doi.org/10.2174/1389450117666160502151707
Y. Pommier, Chem. Rev. 109, 2894 (2009). https://doi.org/10.1021/cr900097c
L. Wang, Z. Li, L. Zhang, et al., Pestic. Biochem. Physiol. 139, 46 (2017). https://doi.org/10.1016/j.pestbp.2017.04.008
M. E. Wall, M. C. Wani, C. E. Cooke, et al., J. Am. Chem. Soc. 88, 3888 (1966). https://doi.org/10.1021/ja00968a057
Y. M. Sun, J. Li, H. Zhao, et al., J. Inorg. Biochem. 163 88 (2016). https://doi.org/10.1016/j.jinorgbio.2016.04.028
G. L. Liao, X. Chen, J. C. Wu, et al., Dalton Trans. 44, 15145 (2015). https://doi.org/10.1039/C4DT03585B
R. Gaur and M. Usman, Spectrochim. Acta Part A. 209, 100 (2019). https://doi.org/10.1016/j.saa.2018.10.035
X. W. Liu, J. Huang, Y. X. Tang, et al., Appl. Organomet. Chem. 32, e4423 (2018). https://doi.org/10.1002/aoc.4423
A. Mushtaq, S. Ali, M. N. Tahir, et al., Russ. J. Inorg. Chem. 64, 1365 (2019). https://doi.org/10.1134/S0036023619110147
Y. C. Liu, Y. Y. Li, H. L. Qi, et al., Russ. J. Coord. Chem. 45, 446 (2019). https://doi.org/10.1134/S1070328419060034
A. K. Todd, S. M. Haider, G. N. Parkinson, et al., Nucleic Acids Res. 35, 5799 (2007). https://doi.org/10.1093/nar/gkm609
J. Dash, P. S. Shirude, S. D. Hsu, et al., J. Am. Chem. Soc. 130, 15950 (2008). https://doi.org/10.1021/ja8046552
K. M. Miller, R. Rodriguez, Expert Rev. Clin. Pharmacol. 4, 139 (2011). https://doi.org/10.1586/ecp.11.4
H.J. Lipps and D. Rhodes, Trends Cell Boil. 19, 414(2009). https://doi.org/10.1016/j.tcb.2009.05.002
S. Balasubramanian, L. H. Hurley, and S. Neidle, Nat. Rev. Drug. Discov. 10, 261 (2011). https://doi.org/10.1038/nrd3428
S. Balasubramanian and S. Neidle, Curr. Opin. Chem. Boil. 13, 345 (2009). https://doi.org/10.1016/j.cbpa.2009.04.637
S. Neidle, FEBS J. 277, 1118(2010). https://doi.org/10.1111/j.1742-4658.2009.07463.x
D. L. Ma, Z. Zhang, M. Wang, et al., Chem. Biol. 22, 812 (2015). https://doi.org/10.1016/j.chembiol.2015.06.016
D. L. Ma, M. Wang, S. Lin, et al., Curr. Top. Med. Chem. 15, 1957 (2015). https://doi.org/10.2174/1568026615666150515150106
B. R. Vummidi, J. Alzeer, and N. W. Luedtke, ChembioChem. 14, 540 (2013). https://doi.org/10.1002/cbic.201200612
M. Wang, Z. F. Mao, T. S. Kang, et al., Chem. Sci. 7, 2516 (2016). https://doi.org/10.1039/C6SC00001K
G. L. Liao, X. Chen, L. N. Ji, et al., Chem. Commun. 48, 10781 (2012). https://doi.org/10.1039/C2CC36039J
D. Saadallah, M. Bellakhal, S. Amor, et al., Chem. Eur. J. 23, 4967 (2017). https://doi.org/10.1002/chem.201605948
L. Xu, D. Zhang, J. Huang, et al., Chem. Commun. 46, 743 (2010). https://doi.org/10.1039/B918045A
L. Li, H. M. Liu, X. K. Liu, et al., RSC Adv. 7, 23727 (2017). https://doi.org/10.1039/c7ra01853c
X. Dang and J. T. Hupp, J. Photochem. Photobiol. A. 143, 251 (2001). https://doi.org/10.1016/S1010-6030(01)00484-1
J. G. Liu, B. H. Ye, H. Chao, et al., Chem. Lett. 28, 1085 (1999). https://doi.org/10.1246/cl.1999.1085
J. D. McGhee and P.H. von Hippel, J. Mol. Biol. 86, 469 (1974). https://doi.org/10.1016/0022-2836(74)90031-X
R. M. Hartshorn and J. K. Barton, J. Am. Chem. Soc. 114, 5919 (1992). https://doi.org/10.1021/ja00041a002
E. A. Steck and A. R. Day, J. Am. Chem. Soc. 65, 452(1943). https://doi.org/10.1021/ja01243a043
L. He, X. Chen, Z. Y. Meng, et al., Chem. Commun. 52, 8095(2016). https://doi.org/10.1039/c6cc03117j
J. L. Yao, X. Gao, W. L. Sun, et al., Inorg. Chem. 51, 12591(2012). https://doi.org/10.1021/ic301305q
G. Shi, S. Monro, R. Hennigar, et al., Coord. Chem. Rev. 282-283, 127(2015). https://doi.org/10.1016/j.ccr.2014.04.012
D. A. Smithen, H. Yin, M. H. R. Beh, et al., Inorg. Chem. 56, 4121(2017). doi. https://doi.org/10.1021/acs.inorgchem.7b00072
R. Nilsson, P. B. Merkel, D.R. Kearns. Photochem. Photobiol. 16, 117(1972). https://doi.org/10.1111/j.1751-1097.1972.tb07343.x
F. Jensen and C. S. Foote, J. Am. Chem. Soc. 109, 1478(1987). https://doi.org/10.1021/ja00239a030
A. A. Abdel-ShaE, P. D. Beer, R. J. Mortimer, et al., J. Phys. Chem. A 104, 192 (2000). https://doi.org/10.1021/jp991876z
Q. X. Zhou, W. H. Lei, C. Li, et al., New J. Chem. 34, 137 (2010). https://doi.org/10.1039/B9NJ00465C
Y. J. Sun, L. . Joyce, N. M. Dickson, et al., Chem. Commun. 46, 2426 (2010). https://doi.org/10.1039/B925574E
X. W. Liu, L. C. Xu, H. Li, et al., J. Mol. Struct. 920, 163 (2009). https://doi.org/10.1016/j.molstruc.2008.10.038
H. J. Yu, S. M. Huang, L. Y. Li, et al., J. Inorg. Biochem. 103, 881 (2009). https://doi.org/10.1016/j.jinorgbio.2009.03.005
H. J. Yu, H. Chao, L. Jiang, et al., Inorg. Chem. Commun. 11, 553 (2008). https://doi.org/10.1016/j.inoche.2008.02.008
X. W. Liu, Y. M. Shen, J. S. Shu, et al., J. Fluoresc. 25, 1527 (2015). https://doi.org/10.1007/s10895-015-1644-8
Funding
This work was supported by the Research Foundation of Education Bureau of Hunan Province (16A145) and State Key Laboratory of Coordination Chemistry (SKLCC1920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Supplementary material
Rights and permissions
About this article
Cite this article
Xue-Wen Liu, Liu, NY., Deng, YQ. et al. Topoisomerase I Inhibition, DNA Photocleavage Activity, and G-Quadruplex DNA ‘Light Switch’ Based on Nitro-Substituted Ruthenium Complexes. Russ. J. Inorg. Chem. 65, 1186–1195 (2020). https://doi.org/10.1134/S0036023620080094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620080094