Abstract
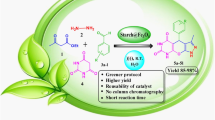
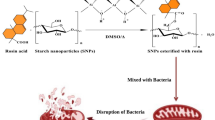
A new method has been proposed for the synthesis of pyranopyrimidinone and xanthene derivatives using zinc oxide–starch nanocomposite as catalyst under microwave irradiation. The ZnO–starch nanocomposite was characterized by X-ray diffraction and scanning electron microscopy data, and the size of the ZnO-starch nanocomposite particles was estimated at 70–90 nm. The catalyst was used in the three-component condensation of aromatic aldehydes with barbituric acid and malononitrile and the 1:2 condensation of aromatic aldehydes with naphthalen-2-ol to obtain pyranopyrimidinone and xanthene derivatives, respectively. The catalyst can be reused several times without loss of catalytic activity. The proposed procedure utilizes affordable and inexpensive materials, provides excellent yields in short reaction time, and is eco-friendly, which makes it more economic than conventional methods. The antibacterial activity of the synthesized compounds was evaluated against M. luteus and P. aeruginosa.






Similar content being viewed by others
REFERENCES
Zolfigol, M.A., Nasrabadi, R.A., and Baghery, S., Appl. Org. Chem., 2016, vol. 30, p. 273. https://doi.org/10.1002/aoc.3428
Pasha, M.A. and Jayashankara, V.P., Bioorg. Med. Chem. Lett., 2007, vol. 17, p. 621. https://doi.org/10.1016/j.bmcl.2006.11.009
Sokolova, N.B., Lisina, I.V., and Tamoshauskas, N.A., Russ. J. Appl. Chem., 2011, vol. 84, p. 670. https://doi.org/10.1134/S1070427211040197
Huang, H., Yao, Y., Lin, Q., Zhao, J., Hua, C., and Gou, X.,Russ. J. Gen. Chem., 2016, vol. 86, p. 934. https://doi.org/10.1134/S1070363216040307
Hanna, M.M., Eur. J. Med. Chem., 2012, vol. 55, p. 12. https://doi.org/10.1016/j.ejmech.2012.06.048
Shinde, S.V., Jadhav, W.N., and Karade, N.N., Orient. J. Chem., 2010, vol. 26, p. 307.
Karimi-Jaberi, Z. and Hashemi, M.M., Monatsh. Chem., 2008, vol. 139, p. 605. https://doi.org/10.1007/s00706-007-0786-z
Lichtner, R.B., Hutchinson, G., and Hellmann, K., Eur. J. Cancer Clin. Oncol., 1989, vol. 25, p. 945. https://doi.org/10.1016/0277-5379(89)90152-1
Casiraghi, G., Casnati, G., and Cornia, M., Tetrahedron Lett., 1973, vol. 14, p. 679. https://doi.org/10.1016/S0040-4039(00)72432-4
Wang, J.Q. and Harvey, R.G., Tetrahedron, 2002, vol. 58, p. 5927. https://doi.org/10.1016/S0040-4020(02)00534-3
Jha, A. and Beal, J., Tetrahedron Lett., 2004, vol. 45, p. 8999. https://doi.org/10.1016/j.tetlet.2004.10.046
Kobayashi, S., Busujima, T., and Nagayama, S., Chem. Eur. J., 2000, vol. 6, p. 3491. https://doi.org/10.1002/1521-3765(20001002)6:19<3491::AID-CHEM3491>3.0.CO;2-P
Rostamizadeh, S., Shadjou, N., Amani, A.M., and Balalaie, S.,Chin. Chem. Lett., 2008, vol. 19, p. 1151. https://doi.org/10.1016/j.cclet.2008.07.026
Soleimani, E., Khodaei, M.M., and Koshvandi, A.T.K., Chin. Chem. Lett., 2011, vol. 22, p. 927. https://doi.org/10.1016/j.cclet.2011.01.012
Gong, K., Fang, D., Wang, H.L., Zhou, X.L., and Liu, Z.L.,Dyes Pigm., 2009, vol. 80, p. 30. https://doi.org/10.1016/j.dyepig.2008.02.011
Hajipour, A.R., Ghayeb, Y., Sheikhan, N., and Ruoho, A.E.,Synlett, 2010, vol. 2010, no. 5, p. 741. https://doi.org/10.1055/s-0029-1219399
Rahmatpour, A., Monatsh. Chem., 2011, vol. 142, p. 1259. https://doi.org/10.1007/s00706-011-0537-z
Ghassamipour, S. and Sardarian, A.R., J. Heterocycl. Chem., 2012, vol. 49, p. 669. https://doi.org/10.1002/jhet.780
Wang, J.Q. and Harvey, R.G., Tetrahedron, 2002, vol. 58, p. 5927. https://doi.org/10.1016/S0040-4020(02)00534-3
Stetter, H., Newer Methods of Preparative Organic Chemistry, Foerst, W., Ed., New York: Academic, 1963, vol. 2, p. 51. https://doi.org/10.1016/B978-0-12-395648-4.50010-6
Kumari, P., Yathindranath, V., and Chauhan, S.M.S., Synth. Commun., 2008, vol. 38, p. 637. https://doi.org/10.1080/00397910701798234
Tankam, T., Srisa, J., Sukwattanasinitt, M., and Wacharasindhu, S.,J. Org. Chem., 2018, vol. 83, p. 11936. https://doi.org/10.1021/acs.joc.8b01824
Balakrishna, C., Kandula, V., Gudipati, R., Yennam, S., Devi, P.U., and Behera, M., Synlett, 2018, vol. 29, p. 1087. https://doi.org/10.1055/s-0036-1591898
Pourshamsian, K., J. Chem. Health Risks, 2018, vol. 8, p. 305. https://doi.org/10.22034/jchr.2018.544457
Montazeri, N., Pourshamsian, K., Rezaei, H., and Fouladi, M.,Asian J. Chem., 2013, vol. 25, p. 3463. dx.doi.org/10.14233/ajchem.2013
Montazeri, N., Pourshamsian, K., Bayazi, M., and Kabiri, S.,Asian J. Chem., 2013, vol. 25, p. 3373. https://doi.org/10.14233/ajchem.2013.13774
Montazeri, N., Pourshamsian, K., Yosefiyan, S., and Momeni, S.S.,J. Chem. Sci., 2012, vol. 124, p. 883. https://doi.org/10.1007/s12039-012-0260-2
Yavari, I., Asgari, S.A., Pourshamsian, K., and Bagheri, M.,J. Sulfur Chem., 2007, vol. 28, p. 477. https://doi.org/10.1080/17415990701471364
Tavakolifard, S., Biazar, E., Pourshamsian, K., and Moslemin, M.H.,Artif. Cells, Nanomed., Biotechnol., 2016, vol. 44, p. 1247. https://doi.org/10.3109/21691401.2015.1019670
Jinxia, M., Wenhua, Z., Yajun, T., and Zhiguo, W., Nanoscale Res. Lett., 2016, vol. 11, p. 200. https://doi.org/10.1186/s11671-016-1404-y
Darder, M., Aranda, P., and Ruiz-Hitzky, E., Adv. Mater., 2007, vol. 19, p. 1309. https://doi.org/10.1002/adma.200602328
Seoyoun, S. and Jyongsik, J., Chem. Commun., 2007, vol. 22, p. 4230. https://doi.org/10.1039/B707706H
Albadi, J., Mansournezhad, A., and Sadeghi, T., Res. Chem. Intermed., 2015, vol. 41, p. 8317. https://doi.org/10.1007/s11164-014-1894-0
Majid, M.H., Hamideh, A., Khadijeh, B., Mina, S., Hossein, A.O., and Fatemeh, F.B., Bull. Chem. Soc. Ethiop., 2011, vol. 25, p. 399. https://doi.org/10.4314/bcse.v25i3.68592
Shaterian, H.R., Doostmohammadi, R., and Ghashang, M., Chin. J. Chem., 2008, vol. 26, p. 338. https://doi.org/10.1002/cjoc.200890065
Fei-Oing, D., Li-Tao, A., and Jian-Ping, Z., Chin. J. Chem., 2007, vol. 25, p. 645. https://doi.org/10.1002/cjoc.200790120
Zakeri, M., Heravi, M., Saeedi, M., Karimi, N., Oskooie, H.A., and Tavakoli, N., Chin. J. Chem., 2011, vol. 29, p. 1441. https://doi.org/10.1002/cjoc.201180240
Wang, L.M., Sui, Y., and Zhang, L., Chin. J. Chem., 2008, vol. 26, p. 1105. https://doi.org/10.1002/cjoc.200890196
Yoshioka, E., Nishimura, M., Nakazawa, T., Kohtani, S., and Miyabe, H., J. Org. Chem., 2015, vol. 80, p. 8464. https://doi.org/10.1021/acs.joc.5b01452
Zang, H., Zhang, Y., Cheng, B., Zhang, W., Xu, X., and Ren, Y.,Chin. J. Chem., 2012, vol. 30, p. 362. https://doi.org/10.1002/cjoc.201180488
Yuling, L., Baixiang, D.U., Xiaoping, X.U., Daqing, S.H.I., and Shunjun, J.I., Chin. J. Chem., 2009, vol. 27, p. 1563. https://doi.org/10.1002/cjoc.200990264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Amininia, A., Pourshamsian, K. & Sadeghi, B. Nano-ZnO Impregnated on Starch—A Highly Efficient Heterogeneous Bio-Based Catalyst for One-Pot Synthesis of Pyranopyrimidinone and Xanthene Derivatives as Potential Antibacterial Agents. Russ J Org Chem 56, 1279–1288 (2020). https://doi.org/10.1134/S1070428020070234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020070234