Abstract
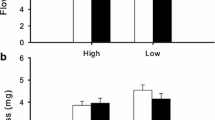
Gynodioecy, the co-occurrence of female and hermaphrodite plants in the same population is relatively common in the genus Thymus, where a high and variable female frequency has been reported in several species, populations and years. In most gynodioecious species, female plants produce more and/or better seeds than hermaphrodite plants, facilitating female maintenance in natural populations. Thymus × arundanus and T. granatensis are two sympatric gynodioecious species that inhabit the region of Andalucia, Spain. Here we studied reproductive components in two different years (1987 and 2016) as flower number, fruit-set, seed number and seed mass, and their possible relation to female frequencies in those years in natural populations of both species. In T. × arundanus, mean female frequency (59%) was ten times higher than that of T. granatensis (5%). Female frequency was relatively constant in T. × arundanus after almost 15 years (1987–2016), and in T. granatensis low female frequencies were observed (0–5%) with the exception of one population (18%). However, reproductive components were variable among species, years and genders, showing no consistent female fertility advantage. High female frequencies in T. × arundanus are not likely to be maintained by female fertility advantage but stochastic and pleiotropic effects on sex determination may play a relevant role in sex ratio variation in the studied populations of both species.



Similar content being viewed by others
References
Alonso C, Herrera CM (2001) Neither vegetative nor reproductive advantages account for high frequency of male-steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae). Am J Bot 88(6):1016–1024
Ashman TL (2002) The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83(5):1175–1184
Aparicio Martinez A, Silvestre Domingo S (1987). Flora del Parque Natural de la Sierra de Grazalema. Junata de Andalucia, Sevilla. .Agencia del Medio Ambiente. Monografías del medio ambiente, 5.
Arroyo J, Marañón T (1990) Community ecology and distributional spectra of Mediterranean shrublands and heathlands in Southern Spain. J Biogeogr 17:163–176
Asikainen E, Mutikainen P (2003) Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae). Am J Bot 90(2):226–234
Belhassen E, Trabaud L, Couvet D, Gouyon PH (1989) An example of nonequilibrium processes: gynodioecy of Thymus vulgaris L. in burned habitats. Evolution 43(3):662–667
Bailey MF, Delph LF (2007) Sex-ratio evolution in nuclear-cytoplasmic gynodioecy when restoration is a threshold trait. Genetics 176(4):2465–2476
Barr CM (2004) Hybridization and regional sex ratios in Nemophila menziessi. J Evol Biol 17:786–794
Campbell DR, Alarcon R, Wu CA (2003) Reproductive isolation and hybrid pollen disadvantage in Ipomopsis. J Evol Biol 16(3):536–540
Caruso CM, Case AL (2013) Testing models of sex ratio evolution in a gynodioecious plant: female frequency covaries with the cost of male fertility restoration. Evolution 67(2):561–566
Crawley MJ (2007) The R book. Wiley, Chichester
Darwin C (1877) The different forms of flowers on plants of the same species. John Murray, London
Cuevas E, Arias DM, Dominguez CA, Castillo RA, Molina-Freaner F (2006) The genetic structure of the gynodioecious Kallstroemia grandiflora (Zygophyllaceae): the role of male sterility and colonization history. Heredity 97(4):269–274
Delph LF, Touzet P, Bailey MF (2007) Merging theory and mechanism in studies of gynodioecy. Trends Ecol Evol 22(1):17–24
Dommée B, Assouad MW, Valdeyron G (1978) Natural selection and gynodioecy in Thymus vulgaris L. Bot J Linn Soc 77(1):17–28
Dufay M, Billard E (2012) How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann Bot 109(3):505–519
Dufay M, Cuguen J, Arnaud JF, Touzet P (2009) Sex ratio variation among gynodioecious populations of sea beet: can it be explained by negative frequency-dependent selection? Evolution 63(6):1483–1497
Frank SA (1989) The evolutionary dynamics of cytoplasmic male sterility. Am Nat 133(3):345–376
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol 45(3):115–120
Heslop-Harrison J, Heslop-Harrison Y, Shivanna KR (1984) The evaluation of pollen quality, and a further appraisal of the fluorochromatic (FCR) test procedure. Theor Appl Genet 67(4):367–375
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Colorado
Klips RA, Culley TM (2004) Natural hybridization between prairie milkweeds, Asclepias sullivantii and Asclepias syriaca: morphological, isozyme, and hand-pollination evidence. Int J Plant Sci 165(6):1027–1037
Lau CP, Ramsden L, Saunders RM (2005) Hybrid origin of Bauhinia blakeana (Leguminosae: Caesalpinioideae), inferred using morphological, reproductive, and molecular data. Am J Bot 92(3):525–533
Manicacci D, Atlan A, Elena Rossello JA, Couvet D (1998) Gynodioecy and reproductive trait variation in three Thymus species (Lamiaceae). Int J Plant Sci 159(6):948–957
McCauley DE, Brock MT (1998) Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution 52(1):30–36
Morales Valverde R (1986) Taxonomía de los géneros Thymus (excluida la sección Serpyllum) y Thymbra en la Península Ibérica. Ruizia 3:1–324
Murayama K, Yahara T, Terachi T (2004) Variation of female frequency and cytoplasmic male-sterility gene frequency among natural gynodioecious populations of wild radish (Raphanus sativus L.). Mol Ecol 13(8):2459–2464
Nilsson E, Ågren J (2006) Population size, female fecundity, and sex ratio variation in gynodioecious Plantago maritima. J Evol Biol 19:825–833
Orellana ME, Rovira AM, Blanché C, Bosch M (2005) Pollination and reproductive success in the gynodioecious endemic Thymus loscosii (Lamiaceae). Can J Bot 83:183–193
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org.
Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot 101(10):1588–1596
Rieseberg LH, Kim SC, Randell RA, Whitney KD, Gross BL, Lexer C, Clay K (2007) Hybridization and the colonization of novel habitats by annual sunflowers. Genetica 129(2):149–165
Rivkin LR, Case AL, Caruso CM (2015) Frequency-dependent fitness in gynodioecious Lobelia siphilitica. Evolution 69(5):1232–1243
Rivkin LR, Case AL, Caruso CM (2016) Why is gynodioecy a rare but widely distributed sexual system? Lessons from the Lamiaceae. New Phytol 211(2):688–696
Shykoff JA, Kolokotronis SO, Collin CL, López-Villavicencio M (2003) Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135(1):1–9
Spigler RB, Ashman TL (2011) Gynodioecy to dioecy: are we there yet? Ann Bot 109(3):531–543
StakelienĖ V, LožienĖ K (2014) Gynodioecy in Thymus pulegioides L, T serpyllum L, and their hybrid T x oblongifolius Opiz (Lamiaceae): flower size dimorphism, female frequency, and effect of environmental factors. Plant Biosyst Int J Dealing Aspects Plant Biol 148(1):49–57
Thompson JD (2002) Population structure and the spatial dynamics of genetic polymorphism in thyme. Thyme 44–74
Van Etten ML, Deen AC, Hamrick JL, Chang SM (2014) Mating system contributes only slightly to female maintenance in gynodioecious Geranium maculatum (Geraniaceae). Heredity 113(5):464
Vaughton G, Ramsey M (2005) Dry environments promote the establishment of females in monomorphic populations of Wurmbea biglandulosa (Colchicaceae). Evol Ecol 18(4):323–341
Weller SG, Sakai AK (2005) Selfing and resource allocation in Schiedea salicaria (Caryophyllaceae), a gynodioecious species. J Evol Biol 18(2):301–308
Acknowledgements
The authors thanks to F. Rosas Pacheco for critical comments on earlier versions of this manuscript and two anonymous reviewers for their valuable comments. G. Cuevas García help to build the map of studied populations. E. Cuevas thanks to the National Council of Science and Technology of Mexico (CONACyT) for the financial support for the Sabbatical stay at the U. Seville, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Siegy Krauss.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cuevas, E., Andrés, M.C. & Arroyo, J. Reproductive biology and female frequencies of two co-occurring gynodioecious Thymus species. Plant Ecol 221, 1243–1251 (2020). https://doi.org/10.1007/s11258-020-01078-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01078-1