Abstract
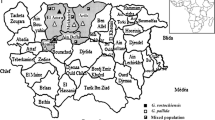
Global trading of plant materials, in combination with agricultural practices, may facilitate the spreading of cyst nematodes to so far non-infected areas. Recently Potato Cyst Nematode (PCN) was recognized to be present in Indonesia and both diversity and distribution require further study. Assessment of PCN populations was done by collecting soil samples, determination of morphological characteristics in combination with ITS rDNA and COI mtDNA sequencing. Thirty-seven soil samples were collected from potato fields in the Indonesia archipelago. The results showed the presence of Globodera rostochiensis in 22 out of 37 sampling fields, namely North Sumatra (6 fields), Central Java (12 fields), East Java (3 fields), and -for the first time- in Sulawesi (North Sulawesi) (1 field). The highest observed density was found in Banjarnegara (Central Java), i.e., 872 cysts 100 ml soil−1. Globodera pallida was not recovered. Both ITS and COI characterisation of Indonesian PCN (G. rostochiensis) revealed the virtual absence of sequence variation as compared to most PCN from the rest of the world; the COI sequences were identical to the most common and mostly distributed haplotype around the world. Microsatellite genotyping indicated a higher genetic diversity for populations from East Java than for populations from North Sumatra, suggesting that cysts at the origin of populations in North Sumatra were coming from populations in East Java. These data on species identification, population density, genetic diversity, and distribution of potato cyst nematode over the Indonesian archipelago constitute the very basis for the design of environmentally-sound and effective PCN control strategies.





Similar content being viewed by others
Notes
Based on estimation of presence of eggs in the Dieng Kulon population. The average of eggs Globodera from Dieng Kulon = 300 eggs/cyst; therefore 872 cyst 100 ml−1 = 872 x ~ 300 eggs 100 ml soil−1 = ~2616 eggs ml soil−1
Conversion dried soil in Dieng Kulon-Banjarnegara from ml to gram: 100 ml = 78 g
References
Alenda, C., Montarry, J., & Grenier, E. (2014). Human influence on the dispersal and genetic structure of French Globodera tabacum populations. Infection, Genetics, and Evolution, 27, 309–317. https://doi.org/10.1016/j.meegid.2014.07.027.
Baunacke, W. (1922). Investigations on biology and control ofBeet nematodes, Heterodera schachtii Schmidt. Arbeiten aus der Biologischen Reichsanstalt Berlin, 11, 185–288.
Been, T. H., & Schomaker, C. H. (2000). Development and evaluation of sampling methods for fields with infestation foci of potato cyst nematodes (Globodera rostochiensis and G. pallida). Phytopathology, 90(6), 647–656. https://doi.org/10.1094/PHYTO.2000.90.6.647.
Belkhir, K., Borsa, P., Chikhi, L., Raufaste, N., & Bonhomme, F. (2004). GENETIX 4.05, logiciel sous windows TM pour la génétique des populations. Laboratoire Génome, populations, interactions, CNRS UMR 5000, Université de Montpellier II, Montpellier (France).
Boucher, A. C., Mimee, B., & Montarry, J. (2013). Genetic diversity of the golden potato cyst nematode Globodera rostochiensis and determination of the origin of populations in Quebec, Canada. Molecular Phylogenetics and Evolution, 69, 75–82. https://doi.org/10.1016/j.ympev.2013.05.020.
Bowles, J., Blair, D., & McManus, D. P. (1992). Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Molecular and Biochemical Parasitology, 54, 165–174.
Castagnone-Sereno, P., Skantar, A., & Robertson, L. (2011). Molecular tools for diagnosis. In J. Jones, G. Gheysen, & C. Fenoll (Eds.), Genomics and molecular genetics of plant nematode interactions (1st ed., pp. 443–464). New York: Springer.
Dawson, P., Adnyana, P. C. P., Ameriana, M., Arifudin, A., Assad, M., Basuki, R. S., Budi, B., Crawford, R., de Boer, R., Donald, C., Effendi, P., Furlong, M., Fiona, G., Gunadi, N., Gunawan, G., Hidayah, B. N., Hidayat, D., Hill, T., Himawan, H., Indarti, S., Istiyanto, E., Jayadi, J., Kumoro, K., Kuswardiyanto, K., Lancaster, R., Learmonth, S., Lolugau, B. A., Marshall, J., Mattingley, P., McPharlin, I., Mufrodin, M., Mukhibah, L., Mulyadi, M., Mulyanto, M., Murtiningsih, R., Mustafa, W., Nasrullah, N., Ning, S. W., Nurjanami, N., Pakih, M., Rahadi, R., Rukmana, J., Rahayu, B., Ridland, P., Sayono, H., Silva, F., Sofiari, E., Sudjudi, A., Suhari, S., Sulistyo, S., Tahir, H., Taylor, A., Tomkins, B., Tooke, D., Triman, B., Van Burgel, A. Wahid, D., Warda, W., Warren, J., Wawan, S., Yunianto, P., & Zamzaini, Z. (2011). Optimising the productivity of the potato/brassica cropping system in central and West Java and potato/brassica/allium system in South Sulawesi and Nusa Tenggara Barat. Final report project AGB/2005/167 (ACIAR Canberra).
Dewantoro. (2017). Sahula Sipayung, Penuhi Kebutuhan Benih Kentang Petani. Medan bisnis daily-online newspaper, 23 January 2017. Available via http //www.mdn.biz.id/n/279610/Sahula Sipayung, Penuhi Kebutuhan Benih Kentang Petani. Accessed 15 January 2020.
Earl, D. A., & vonHoldt, B. M. (2012). STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, 4, 359–361. https://doi.org/10.1007/s12686-011-9548-7.
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797. https://doi.org/10.1093/nar/gkh340.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Molecular Ecology, 14, 2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x.
Falush, D., Stephens, M., & Pritchard, J. K. (2003). Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics, 164, 1567–1587.
Floyd, R., Abebe, E., Papert, A., & Blaxter, M. (2002). Molecular barcodes for soil nematode identification. Molecular Ecology, 11, 839–850.
Gautier, C., Esquibet, M., Fournet, S., Piriou, C., Yvin, J.-C., Nguema-Ona, E., Grenier, E., & Montarry, J. (2019). Microsatellite markers reveal two genetic groups in European populations of the carrot cyst nematode Heterodera carotae. Infection, Genetics and Evolution, 73, 81–92. https://doi.org/10.1016/j.meegid.2019.04.011.
Hadisoeganda, A. W. W. (2006). Nematoda Sista Kentang: Kerugian, Deteksi, Biogeografi, dan Pengendalian Nematoda Terpadu. Monografi, 29, 1–52.
Hajihassani, A., Ebrahimian, E., & Hajihasani, M. (2013). Estimation of yield damage in potato caused by the Iranian population of Globodera rostochiensis with and without Aldicarb under greenhouse conditions. International Journal of Agriculture and Biology, 15, 352–356.
Handoo, Z. A., Carta, L. K., Skantar, A. M., & Chitwood, D. J. (2012). Description of Globodera ellingtonae n. sp. (Nematoda: Heteroderidae) from Oregon. Journal of Nematology, 44(1), 40–57.
Hu, M., Chilton, N. B., Zhu, X., & Gasser, R. B. (2002). Single-strand conformation polymorphism-based analysis of mitochondrial cytochrome c oxidase subunit 1 reveals significant substructuring in hookworm populations. Electrophoresis, 23, 27–34.
Huelsenbeck, J. P., & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8), 754–755.
Indarti, S., Rahayu, B., Mulyadi, M., & Triman, B. (2004). Disease notes or new records: First record of potato cyst nematode Globodera rostochiensis in Indonesia. Australasian Plant Pathology, 33, 325–326.
Jarne, P., & Lagoda, P. J. L. (1996). Microsatellites, from molecules to populations and back. Tree, 11(10), 424–429. https://doi.org/10.1016/0169-5347(96)10049-5.
Jones, J., Gheysen, G., & Fenoll, C. (Eds.). (2011). Genomics and molecular genetics of plant-nematode interactions. Springer: London & New York.
Jones, J. T., Haegeman, A., Geraert, E., Danchin, J., Hari, S., Gaur, H. J., Jones, M. G. K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M. L., & Perry, R. N. (2013). Review top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology., 14, 946–961. https://doi.org/10.1111/mpp.12057.
Jones, L. M., Koehler, A. -K., Trnka, M., Balek, J., Challinor, A. J., Atkinson, H. J., & Urwin, P. E. (2017). Climate change is predicted to alter the current pest status of Globodera pallida and G. rostochiensis in the United Kingdom. Global Change Biology, 23, 4497–4507. https://doi.org/10.1111/gcb.13676.
Kaczmarek, A., MacKenzie, K., Kettle, H., & Blok, V. C. (2014). Influence of soil temperature on Globodera rostochiensis and Globodera pallida. Phytopathologia Mediterranea, 53(3), 396–405. https://doi.org/10.14601/Phytopathol_Mediterr-13512.
Langella, O. (1999). Populations, 1.2.32. Available via http://bioinformatics.org/~~tryphon/populations/
Lisnawita, L., Supramana, S., & Suastika, G. (2012). Identification of potato cyst nematode in Indonesia. Australasian Plant Disease Notes, 7, 133–135. https://doi.org/10.1007/s13314-012-0067-5.
Lax, P., Dueñas, J. C. R., Franco-Ponce, J., Gardenal, C. N., & Doucet, M. E. (2014). Morphology and DNA sequence data reveal the presence of Globodera ellingtonae in the Andean region. Zoology, 83(4), 227–243.
Madani, M., Subbotin, S. A., Ward, L. J., Li, X., & De Boer, S. H. (2010). Molecular characterization of Canadian populations of potato cyst nematodes. Globodera rostochiensis and G. pallida using ribosomal nuclear RNA and cytochrome b genes. Canadian Journal of Plant Pathology, 32(2), 252–263. https://doi.org/10.1080/07060661003740033.
Mugniéry, D. & Phillips, M. S. (2007). The Nematode Parasites of Potato. In: Vreugdenhil, D. (Editor). 2007. Potato Biology and Biotechnology: Advances and Perspectives. Elsevier B.V., pp. 469–574.
Mulyadi, M., Rahayu, B., Triman, B., & Indarti, S. (2003). Identification of golden potato cyst nematode (Globodera rostochiensis) in Batu, East Java. Jurnal Perlindungan Tanaman Indonesia, 9(1), 46–53.
Mulyadi, M., Triman B., Indarti, S., Murti, R. H. & Rahayu, B. (2005). The effect of initial population levels of Globodera rostochiensis on the yield of potato. International Conference of Crop Security. Brawijaya University, Malang, East Java Indonesia, September 20–25.
Mulyadi, M., Indarti, S., Rahayu, B., & Triman, B. (2014). Molecular and pathotype identification of potato cyst nematodes. Identifikasi molekuler dan patotipe nematoda sista kentang. Jurnal Perlindungan Tanaman Indonesia, 18(1), 17–23.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89(3), 583–590.
Nguyen, H. T., Trinh, Q. P., Couvreur, M., Singh, P. R., Decraemer, W., & Bert, W. (2019). Molecular and morphological characterisation of a new root-lesion nematode, Pratylenchus horti n. sp. (Tylenchomorpha: Pratylenchidae), from Ghent University botanical garden. Nematology, 21, 739–752. https://doi.org/10.1163/15685411-00003249.
Nugrahana, H. C., Indarti S., & Martono, E. (2017). Potato cyst nematode in East Java: Newly infected areas and identification. Nematoda sista kentang di Jawa Timur: Daerah sebaran baru dan identifikasi. Jurnal Perlindungan Tanaman Indonesia, 21(2), 87-95. DOI: https://doi.org/10.22146/jpti.25498.
Nurjanah, N., Trisyono, Y. A., Indarti, S., & Hartono, S. (2016). Identification, distribution and genetic diversity of the golden potato cyst nematode (Globodera rostochiensis) in Java Indonesia. AIP Conference Proceedings 1755, 130006. DOI: https://doi.org/10.1063/1.4958550.
OEPP/EPPO. (2013). PM 7/119 (1) nematode extraction. European and Mediterranean plant protection organization. Bulletin OEPP/EPPO, 43(3), 471–495. https://doi.org/10.1111/epp.12077.
OEPP/EPPO. (2017). PM 7/40 (4) Globodera rostochiensis and Globodera pallida. European and Mediterranean plant protection organization. Bulletin OEPP/EPPO, 47(2), 174–197. ISSN 0250-8052. https://doi.org/10.1111/epp.12391.
Phillips, M. S. (1989). The role of cyst nematodes in crop rotations in potato. In: J. Vos et al. (eds). Effects of Crop Rotation on Potato Production in the Temperate Zones. Uy Kluwer academic publishers, pp. 95-109.
Picard, D., Plantard, O., Scurrah, M., & Mugniéry, D. (2004). Inbreeding and population structure of the potato cyst nematode (Globodera pallida) in its native area (Peru). Molecular Ecology, 13(10), 2899–2908. https://doi.org/10.1111/j.1365-294X.2004.02275.x.
Picard, D., Sempere, T., & Plantard, O. (2007). A northward colonisation of the Andes by the potato cyst nematode during geological times suggests multiple host-shifts from wild to cultivated potatoes. Molecular Phylogenetics and Evolution, 42, 308–316. https://doi.org/10.1016/j.ympev.2006.06.018.
Plantard, O., & Porte, C. (2004). Population genetic structure of the sugar beet cyst nematode Heterodera schachtii: A gonochoristic and amphimictic species with highly inbred but weakly differentiated populations. Molecular Ecology, 13, 33–41.
Plantard, O., Picard, D., Valette, S., Scurrah, M., Grenier, E., & Mugniéry, D. (2008). Origin and genetic diversity of Western European populations of the potato cyst nematode (Globodera pallida) inferred from mitochondrial sequences and microsatellite loci. Molecular Ecology, 17, 2208–2218. https://doi.org/10.1111/j.1365-294X.2008.03718.x.
Pritchard, J. K., Stephens, M., & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155, 945–959.
Reid, A., & Pickup, J. (2005). Molecular characterization of a morphologically unusual potato cyst nematode. OEPP/EPPO Bulletin, 35, 69–72.
Schlötterer, C. (2000). Evolutionary dynamics of microsatellite DNA. Chromosoma, 109, 365–371. https://doi.org/10.1007/s004120000089.
Selkoe, A. K., & Toonen, R. J. (2006). Microsatellites for ecologists: A practical guide to using and evaluating microsatellite markers. Ecology Letters, 9, 615–629. https://doi.org/10.1111/j.1461-0248.2006.00889.x.
Southey, J. F. (1974). Methods for detection of potato cyst nematodes. EPPO Bulletin, 4(4), 463–473. https://doi.org/10.1111/j.1365-2338.1974.tb02394.x.
Subbotin, S. A., Vierstraete, A., De Ley, P., Rowe, J., Waeyenberge, L., Moens, M., & Vanfleteren, J. R. (2001). Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Molecular Phylogenetics and Evolution, 21(1), 1–16. https://doi.org/10.1006/mpev.2001.0998.
Subbotin, S.A, Mundo-Ocampo, M., & Baldwin, J. G. (2010). Hunt D., J. And Perry R., N. (series editors). Nematology monographs and perspectives volume 8A. Systematics of Cyst Nematodes (Nematoda: Heteroderinae). The Netherlands, Brill Academic Publishers, Martinus Nijhoff Publishers and VSP.
Subbotin, S. A., Toumi, F., Elekçioğlu, I. H., Waeyenberge, L., & Tanha Maafi, Z. (2018). DNA barcoding, phylogeny, and phylogeography of the cyst nematode species of the Avenae group from the genus Heterodera (Tylenchida: Heteroderidae). Nematology, 20(7), 671–702. https://doi.org/10.1163/15685411-00003170.
Subbotin, S. A., Franco, J., Knoetze, R., Roubtsova, T. V., Bostock, R. M., & Cid Del Prado Vera, I. (2020). DNA barcoding, phylogeny and phylogeography of the cyst nematode species from the genus Globodera (Tylenchida: Heteroderidae). Nematology, 22(3), 269–297. https://doi.org/10.1163/15685411-00003305.
Syafii, D. S., Lisnawita, L., & Hasanudin, H. (2018). Sebaran nematoda sista kentang di Wonosobo dan Banjarnegara, Jawa Tengah. Distribution of potato cyst nematode in Wonosobo and Banjarnegara, Central Java. Jurnal Fitopatologi Indonesia, 14(4), 111–119. https://doi.org/10.14692/jfi.14.4.111.
Tanha Maafi, Z., Subbotin, S. A., & Moens, M. (2003). Molecular identification of cyst-forming nematodes (Heteroderidae) from Iran and a phylogeny based on ITS-rDNA sequences. Nematology, 5(1), 99–111. https://doi.org/10.1163/156854102765216731.
Thiéry, M., & Mugniéry, D. (2000). Microsatellite loci in the phytoparasitic nematode Globodera. Genome, 43, 160–165.
Turner, S. J., & Subbotin, S. A. (2013). Cyst nematodes. In R. N. Perry & M. Moens (Eds.), Plant nematology (2nd ed., pp. 109–143). Wallingford: CAB International.
Wang, H.-M., Zhao, H. H., & Chu, D. (2015). Genetic structure analysis of populations of the soybean cyst nematode, Heterodera glycines, from North China. Nematology, 17(5), 591–600. https://doi.org/10.1163/15685411-00002893.
Wang, J. (2017). The computer program STRUCTURE for assigning individuals to populations: Easy to use but easier to misuse. Molecular Ecology Resources, 17(5), 981–990. https://doi.org/10.1111/1755-0998.12650.
Wang, X., Ma, J., Liu, H., Liu, R., & Li, H. (2018). Development and characterization of EST-derived SSR markers in the cereal cyst nematode Heterodera avenae. European Journal of Plant Pathology, 150(1), 105–113. https://doi.org/10.1007/s10658-017-1256-z.
Weir, B. S., & Cockerham, C. C. (1984). Estimating F-statistics for the analysis of population structure. Evolution, 38(6), 1358–1370.
Wollenweber, H. W. (1923). Krankheiten und Beschädigungen der Kartoffel. Arbeiten Forschungs Institut für Kartoffelbau Berlin, 7, 1–56.
Acknowledgements
This work was funded by the Ministry of Agriculture, Nature and Food Quality, The Netherlands (project number 1300023185), in collaboration with the Indonesian Agricultural Quarantine Agency (IAQA) and Ghent University Belgium. The authors would like to thank Plant Quarantine of IAQA officers in Medan, Padang, Semarang, Surabaya, Lombok, Makassar, Manado, Mr. Kristiadi from Indonesian Soil Research Institute, and Mr. Rusli from Food Crop Agriculture and Horticulture Service-North Sumatra for assistance on soil sampling.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals
The present research did not involve any experimentation on humans or animals.
Informed consent
All the author certify that the work carried out in this research followed the principles of ethical and professional conduct have been followed. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Handayani, N.D., Esquibet, M., Montarry, J. et al. Distribution, DNA barcoding and genetic diversity of potato cyst nematodes in Indonesia. Eur J Plant Pathol 158, 363–380 (2020). https://doi.org/10.1007/s10658-020-02078-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02078-7