Abstract
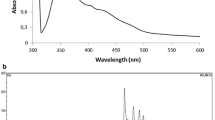
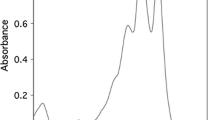
Even though organisms with squalene hopene cyclase activity involved in hopanoid synthesis has been reported earlier, their existence along with carotenoid synthesis is rarely reported. Here, we report the existence of hopanoid and C30 carotenoid biosynthetic pathway in Pseudomonas mendocina, the squalene hopene cyclase producing endophyte of the medicinal plant Murraya koenigii. The enzyme squalene hopene cyclase from Pseudomonas mendocina is involved in the synthesis of dehydrosqualene-mediated alternate pathway for carotenoid biosynthesis. The hopanoids are involved in membrane stability and integrity, and the carotene chromophores are involved in the photo protection of the cell. The orange-colored C30 carotenoid pigment 4–4′ diaponeurosporenic acid in the extracellular extract of Pseudomonas mendocina with squalene cyclase activity was detected by the combination of UV/Vis spectrometry, FTIR, and Mass Spectrometry. 4–4′ diaponeurosporenic acid could be traced as the end product of the carotenoid pathway and belonged to the xanthophyll group of carotenoids.



Similar content being viewed by others
References
Taylor R (1984) Bacterial triterpenoids. Microbiol Mol Biol Rev 48:181–198
Suzue G, Tsukada K, Nakai C, Tanaka S (1968) Presence of squalene in Staphylococcus. Arch Biochem Biophys 123:644
Kleinig H, Schmitt R, Meister W, Englert G, Thommen H et al (1979) New C30-carotenoic acid glucosyl esters from Pseudomonas rhodos. Z Naturforsch. https://doi.org/10.1515/znc-1979-3-404
Tao L, Schenzle A, Odom J (2005) Novel carotenoid oxidase involved in biosynthesis of 4, 4′-diapolycopene dialdehyde. Appl Environ Microbiol 71:3294–3301. https://doi.org/10.1128/AEM.71.6.3294
Steiger S, Perez-Fons L, Fraser PD et al (2012) Biosynthesis of a novel C 30 carotenoid in Bacillus firmus isolates. J Appl Microbiol 113:888–895. https://doi.org/10.1111/j.1365-2672.2012.05377.x
Mohammadi M, Burbank L, Roper MC (2012) biological role of pigment production for the bacterial phytopathogen Pantoea stewartii subsp. stewartii. Appl Environ Microbiol 78:6859–6865. https://doi.org/10.1128/AEM.01574-12
Pelz A, Wieland KP, Putzbach K et al (2005) Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem 280:32493–32498. https://doi.org/10.1074/jbc.M505070200
Ochs D, Kaletta C, Entian KD et al (1992) Cloning, expression, and sequencing of squalene-hopene cyclase, a key enzyme in triterpenoid metabolism. J Bacteriol 174:298–302. https://doi.org/10.1128/jb.174.1.298-302.1992
Ochs D, Tappe CH, Gärtner P et al (1990) Properties of purified squalene-hopene cyclase from Bacillus acidocaldarius. Eur J Biochem 194:75–80. https://doi.org/10.1111/j.1432-1033.1990.tb19429.x
Kleemann G, Kellner R, Poralla K (1994) Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochim Biophys Acta (BBA)/Lipids Lipid Metab 1210:317–320. https://doi.org/10.1016/0005-2760(94)90235-6
Perzl M, Müller P, Poralla K, Kannenberg EL (1997) Squalene-hopene cyclase from Bradyrhizobium japonicum : cloning, expression, sequence analysis and comparison to other triterpenoid cyclases. Microbiology 143:1235–1242. https://doi.org/10.1099/00221287-143-4-1235
Tippelt A, Jahnke L, Poralla K (1998) Squalene-hopene cyclase from Methylococcus capsulatus (Bath): a bacterium producing hopanoids and steroids. Biochim Biophys Acta 1391:223–232. https://doi.org/10.1016/s0005-2760(97)00212-9
Ghimire GP, Oh T-J, Lee HC, Sohng JK (2009) Squalene-hopene cyclase (Spterp25) from Streptomyces peucetius: sequence analysis, expression and functional characterization. Biotechnol Lett 31:565–569. https://doi.org/10.1007/s10529-008-9903-2
Pacifico D, Squartini A, Crucitti D et al (2019) The role of the endophytic microbiome in the grapevine response to environmental triggers. Front Plant Sci 10:1–15. https://doi.org/10.3389/fpls.2019.01256
Fidan O, Zhan J (2019) Discovery and engineering of an endophytic Pseudomonas strain from Taxus chinensis for efficient production of zeaxanthin diglucoside. J Biol Eng 13:1–18. https://doi.org/10.1186/s13036-019-0196-x
Nair MI, Jayachandran K (2017) A novel strain of Pantoea eucrina endophyte of Murraya koenigii with squalene cyclase activity. LIFE Int J Heal Life Sci 3:161–177. https://doi.org/10.20319/lijhls.2017.32.161177
Nair IM, Kochupurakal J (2019) In silico characterization and over-expression of squalene hopene cyclase from Pseudomonas mendocina. 3 Biotech 9:1–7. https://doi.org/10.1007/s13205-019-1901-7
Venil CK, Zakaria ZA, Usha R, Ahmad WA (2014) Isolation and characterization of flexirubin type pigment from Chryseobacterium sp. UTM-3T. Biocatal Agric Biotechnol 3:103–107. https://doi.org/10.1016/j.bcab.2014.02.006
Cornelis P (2010) Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol 86:1637–1645. https://doi.org/10.1007/s00253-010-2550-2
Rohmer M, Bouvier-Nave P, Ourisson G (1984) Distribution of Hopanoid triterpenes in Prokaryotes. Microbiology 130:1137–1150. https://doi.org/10.1099/00221287-130-5-1137
Ku B, Jeong JC, Mijts BN et al (2005) Preparation, characterization, and optimization of an in vitro C30 carotenoid pathway. Appl Env Microbiol 71:6578–6583. https://doi.org/10.1128/AEM.71.11.6578-6583.2005
Wieland B, Feil C, Gloria-Maercker E et al (1994) Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4’-diaponeurosporene of Staphylococcus aureus. J Bacteriol 176:7719–7726. https://doi.org/10.1128/jb.176.24.7719-7726.1994
Kim SH, Kim MS, Lee BY, Lee PC (2016) Generation of structurally novel short carotenoids and study of their biological activity. Sci Rep. https://doi.org/10.1038/srep21987
Acknowledgements
The current study was supported by KSCSTE-SARD Programme, Kerala State Council for Science, Technology and Environment, DBT-MSUB Common Instrumentation Facility, School of Biosciences, and LC-MS/MS facility at Inter University Instrumentation Facility, School of Environmental Sciences, Mahatma Gandhi University Kottayam.
Author information
Authors and Affiliations
Contributions
The author IMN has contributed to the execution of the work and the preparation of the manuscript. The corresponding author JK contributed to the planning of the work and correction of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nair, I.M., Jayachandran, K. 4–4′ Diaponeurosporenic Acid, the C30 Carotenoid Pigment in Endophytic Pseudomonas Mendocina with Squalene Cyclase Activity. Curr Microbiol 77, 3473–3479 (2020). https://doi.org/10.1007/s00284-020-02180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02180-3