Abstract
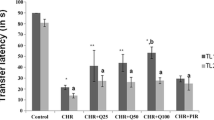
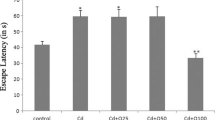
Chromium is a micronutrient which has found frequent use as supplements during pregnancy and could have a role in altering the antioxidant status in the brain. The present study was undertaken to estimate chromium levels in the brain, antioxidant enzyme activity with their gene expression, and learning and memory parameters on F1 and F2 generation mice when the F0 was exposed to chromium. The chromium levels in the brain were estimated using atomic absorption spectrophotometer. The enzyme activity of glutathione-s-transferase (GST) and catalase (CAT) was estimated and their gene expression was evaluated using RT-PCR. The spatial memory was tested using Morris water maze. The learning and recall memory was tested using the step down latency paradigm. The chromium levels were significantly raised in animals treated with Cr per se in F1 generation and quercetin cotreatment reduced the Cr levels in brain significantly. The enzyme activity of GST was significantly less in Cr-treated animals of both generations and this effect was significantly reversed on cotreatment with quercetin. The gene expression of GST matched the enzyme activity. However, catalase activity did not show significant decrease with Cr but cotreatment with quercetin resulted in significant decrease compared with control and this effect was not matched by its gene expression. We observed no significant change in learning and memory parameters in both generations following Cr exposure. Thus, this study demonstrates that chromium exposure in gestation causes changes in enzyme activity especially GST and this change was matched by change in gene expression in GST but not CAT. There was no effect on memory at the given dose.







Similar content being viewed by others
References
Piotrowska A, Pilch W, Tota Ł, Nowak G (2018) Biological significance of chromium III for the human organism. Med Pr 69:211–223. https://doi.org/10.13075/mp.5893.00625
Tang XL, Sun Z, Gong L (2018) Chromium supplementation in women with polycystic ovary syndrome: systematic review and meta-analysis. J Obstet Gynaecol Res 44:134–143. https://doi.org/10.1111/jog.13462
Vincent JB (2013) Chromium: is it essential, pharmacologically relevant, or toxic? In: Sigel A, Sigel H, Sigel R (eds) Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences, vol 13. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7500-8_6
Gultepe EE, Uyarlar C, Bayram İ (2018) Supplementation of Cr methionine during dry period of dairy cows and its effect on some production and biochemical parameters during early lactation and on immunity of their offspring. Biol Trace Elem Res 186:143–153. https://doi.org/10.1007/s12011-018-1279-0
Bailey MM, Sturdivant J, Jernigan PL, Townsend MB, Bushman J, Ankareddi I, Rasco JF, Hood RD, Vincent JB (2008) Comparison of the potential for developmental toxicity of prenatal exposure to two dietary chromium supplements, chromium picolinate and [Cr3O(O2CCH2CH3)(6(H2O)3]+, in mice. Birth Defects Res B Dev Reprod Toxicol 83:27–31. https://doi.org/10.1002/bdrb.20140
San Mauro-Martin I, Ruiz-León AM, Camina-Martín MA, Garicano-Vilar E, Collado-Yurrita L, de Mateo-Silleras B, de Paz Redondo Del Río M (2016) Chromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: a meta-analysis of randomized controlled trials. Nutr Hosp 33:27. https://doi.org/10.20960/nh.v33i1.27
Lin CC, Huang YL (2015) Chromium, zinc and magnesium status in type 1 diabetes. Curr Opin Clin Nutr Metab Care 18:588–592. https://doi.org/10.1097/MCO.0000000000000225
Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD (2010) Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci 13:116–122. https://doi.org/10.1179/147683010X12611460764084
Andersson MA, Petersson Grawé KV, Karlsson OM, Abramsson-Zetterberg LA, Hellman BE (2007) Evaluation of the potential genotoxicity of chromium picolinate in mammalian cells in vivo and in vitro. Food Chem Toxicol 45:1097–1106. https://doi.org/10.1016/j.fct.2006.11.008
Mishra M, Sharma A, Negi MP, Dwivedi UN, Chowdhuri DK (2011) Tracing the tracks of genotoxicity by trivalent and hexavalent chromium in Drosophila melanogaster. Mutat Res 722:44–51. https://doi.org/10.1016/j.mrgentox.2011.02.010
Peng Y, Hu J, Li Y, Zhang B, Liu W, Li H, Zhang H, Hu C, Chen X, Xia W, Lu S, Xu S (2018) Exposure to chromium during pregnancy and longitudinally assessed fetal growth: findings from a prospective cohort. Environ Int 121:375–382. https://doi.org/10.1016/j.envint.2018.09.003
Xia W, Hu J, Zhang B, Li Y, Wise JP Sr, Bassig BA, Zhou A, Savitz DA, Xiong C, Zhao J, Zhou Y (2016) A case-control study of maternal exposure to chromium and infant low birth weight in China. Chemosphere 144:1484–1489. https://doi.org/10.1016/j.chemosphere.2015.10.006
Lee H, Chun JH, Moon DH, Lee CU, Kang SG, Son BC, Kim DH, Lee CH, Kim JW, Lee CK (2004) Effects of chromium (VI) exposure on the placental function and reproduction in rats. J Prev Med Public Health 37:157–165
Halder S, Kar R, Mehta AK, Bhattacharya SK, Mediratta PK, Banerjee BD (2016) Quercetin modulates the effects of chromium exposure on learning, memory and antioxidant enzyme activity in F1 generation mice. Biol Trace Elem Res 171:391–398. https://doi.org/10.1007/s12011-015-0544-8
Cauley M, Hall BJ, Abreu-Villaça Y, Junaid S, White H, Kiany A, Slotkin TA, Levin ED (2018) Critical developmental periods for effects of low-level tobacco smoke exposure on behavioral performance. Neurotoxicology 68:81–87. https://doi.org/10.1016/j.neuro.2018.07.012
Frankel S, Medvedeva N, Gutherz S, Kulick C, Kondratyev A, Forcelli PA (2016) Comparison of the long-term behavioral effects of neonatal exposure to retigabine or phenobarbital in rats. Epilepsy Behav 57:34–40. https://doi.org/10.1016/j.yebeh.2016.01.018
Kawamura S, Yoshioka T, Mito N, Kishimoto N, Nakaoka M, Fantel AG (2016) Mechanism of developmental effects in rats caused by an N-phenylimide herbicide: transient fetal anemia and sequelae during mid-to-late gestation. Birth Defects Res B Dev Reprod Toxicol 107:45–59. https://doi.org/10.1002/bdrb.21172
Halder S, Kar R, Chakraborty S, Bhattacharya SK, Mediratta PK, Banerjee BD (2019) Cadmium level in brain correlates with memory impairment in F1 and F2 generation mice: improvement with quercetin. Environ Sci Pollut Res Int 26:9632–9639. https://doi.org/10.1007/s11356-019-04283-2
Lavado LK, Zhang MH, Patel K, Khan S, Patel UK (2019) Biometals as potential predictors of the neurodegenerative decline in Alzheimer's disease. Cureus 11:e5573. https://doi.org/10.7759/cureus.5573
Oliveira LF, Rodrigues LD, Cardillo GM, Nejm MB, Guimarães-Marques M, Reyes-Garcia SZ, Zuqui K, Vassallo DV, Fiorini AC, Scorza CA, Scorza FA (2020) Deleterious effects of chronic mercury exposure on in vitro LTP, memory process, and oxidative stress. Environ Sci Pollut Res Int 27:7559–7569. https://doi.org/10.1007/s11356-019-06625-6
Anderson RA (1981) Nutritional role of chromium. Sci Total Environ 17:13–29. https://doi.org/10.1016/0048-9697(81)90104-2
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229. https://doi.org/10.1016/j.biopha.2016.12.105
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Borowska S, Brzoska MM, Tomczyk M (2018) Complexation of bioelements and toxic metals by polyphenolic compounds–implications for health. Curr Drug Targets 19:1612–1638. https://doi.org/10.2174/1389450119666180403101555
Cho JY, Kim IS, JangYH KAR, Lee SR (2006) Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global ischaemia. Neurosci Lett 404:330–335. https://doi.org/10.1016/j.neulet.2006.06.010
Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M (2007) Neuroprotective effect of quercetin and rutin on spatial memory impairment in 8-arm radial maze task and neuronal death induced by repeated cerebral ischaemia in rats. J Pharmacol Sci 104:329–334. https://doi.org/10.1254/jphs.fp0070247
Liu J, Yu H, Ning X (2006) Effect of quercetin on chronic enhancement of spatial learning and memory of mice. Sci China C Life Sci 49:583–590. https://doi.org/10.1007/s11427-006-2037-7
Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ (2006) Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res 171:251–260. https://doi.org/10.1016/j.bbr.2006.03.043
Morales AI, Vicente-Sánchez C, Sandoval JM et al (2006) Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol 44:2092–2100. https://doi.org/10.1016/j.fct.2006.07.012
Vicente-Sánchez C, Egido J, Sánchez-González PD, Pérez-Barriocanal F, López-Novoa JM, Morales AI (2008) Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol 46:2279–2287. https://doi.org/10.1016/j.fct.2008.03.009
Kıyga E, Şengelen A, Adıgüzel Z, Önay Uçar E (2020) Investigation of the role of quercetin as a heat shock protein inhibitor on apoptosis in human breast cancer cells. Mol Biol Rep 47:4957–4967. https://doi.org/10.1007/s11033-020-05641-x
Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, Banerjee UC, Kai G (2020) Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in SD rats. Front Bioeng Biotechnol 8:631. https://doi.org/10.3389/fbioe.2020.00631
Morris RG, Garrud P, Rawlins JA, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683. https://doi.org/10.1038/297681a0
Brandeis R, Brandys Y, Yehuda S (1989) The use of the Morris water maze in the study of memory and learning. Int J Neurosci 48:29–69. https://doi.org/10.3109/00207458909002151
Ukai M, Miura M, Kameyama T (1995) Effects of galanin on passive avoidance response, elevated plus-maze learning, and spontaneous alternation performance in mice. Peptides 16:1283–1286. https://doi.org/10.1016/0196-9781(95)02009-l
Mannervik B, Helena Danielson U, Ketterer B (1988) Glutathione transferases—structure and catalytic activit. Crit Rev Biochem 23:283–337. https://doi.org/10.3109/10409238809088226
Luck H (1974) Estimation of catalase activity. In: Bergmeyer U (ed) Methods of enzymology. Academic Press, New York, p 885
Chakraborty P, Jayachandran S, Chakraborty S (2019) Chromium speciation in the sediments across the oxygen minimum zone, western continental margin of India. Geol J 54:1132–1140. https://doi.org/10.1016/j.scitotenv.2016.05.125
Orhan C, Şahin N, Tuzcu Z, Komorowski JR, Şahin K (2017) Combined oral supplementation of chromium picolinate, docosahexaenoic acid, and boron enhances neuroprotection in rats fed a high-fat diet. Turk J Med Sci 47:1616–1625. https://doi.org/10.3906/sag-1701-54
Sahin N, Hayirli A, Orhan C et al (2017) Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult Sci 96:4317–4324. https://doi.org/10.3382/ps/pex249
Cardoso OO, Julião FC, Alves RI, Baena AR, Díez IG, Suzuki MN, Celere BS, Nadal M, Domingo JL, Segura-Muñoz SI (2014) Concentration profiles of metals in breast milk, drinking water, and soil: relationship between matrices. Biol Trace Elem Res 160:116–122. https://doi.org/10.1007/s12011-014-0030-8
Samuel JB, Stanley JA, Roopha DP, Vengatesh G, Anbalagan J, Banu SK, Aruldhas MM (2011) Lactational hexavalent chromium exposure-induced oxidative stress in rat uterus is associated with delayed puberty and impaired gonadotropin levels. Hum Exp Toxicol 30:91–101. https://doi.org/10.1177/0960327110364638
Zheng W, Ge F, Wu K, Chen X, Li X, Chen Y, Lv Y, Lian Q, Ge RS (2018) In utero exposure to hexavalent chromium disrupts rat fetal testis development. Toxicol Lett 299:201–209. https://doi.org/10.1016/j.toxlet.2018.10.010
Loumbourdis NS, Kostaropoulos I, Theodoropoulou B, Kalmanti D (2007) Heavy metal accumulation and metallothionein concentration in the frog Rana ridibunda after exposure to chromium or a mixture of chromium and cadmium. Environ Pollut 145:787–792. https://doi.org/10.1016/j.envpol.2006.05.011
Espart A, Artime S, Tort-Nasarre G, Yara-Varón E (2018) Cadmium exposure during pregnancy and lactation: materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics 10:1359–1367. https://doi.org/10.1039/c8mt00174j
Liu Y, Luo X, Yang C, Yang T, Zhou J, Shi S (2016) Impact of quercetin-induced changes in drug-metabolizing enzyme and transporter expression on the pharmacokinetics of cyclosporine in rats. Mol Med Rep 14:3073–3085. https://doi.org/10.3892/mmr.2016.5616
Jiang W, Hu M (2012) Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv 2:7948–7963. https://doi.org/10.1039/C2RA01369J
Alvarez AI, Real R, Pérez M, Mendoza G, Prieto JG, Merino G (2010) Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci 99:598–617. https://doi.org/10.1002/jps.21851
Gogoi K, Manna P, Dey T, Kalita J, Unni BG, Ozah D, Baruah PK (2019) Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes. Toxicol in Vitro 54:269–279. https://doi.org/10.1016/j.tiv.2018.10.010
Kostaropoulos I, Kalmanti D, Theodoropoulou B, Loumbourdis NS (2005) Effects of exposure to a mixture of cadmium and chromium on detoxification enzyme (GST, P450-MO) activities in the frog Rana ridibunda. Ecotoxicology 14:439–447. https://doi.org/10.1007/s10646-004-1349-2
Sawicka E, Długosz A (2017) The role of 17β-estradiol metabolites in chromium-induced oxidative stress. Adv Clin Exp Med 26:215–221. https://doi.org/10.17219/acem/62217
Mohamed AA, El-Houseiny W, El-Murr AE, Ebraheim LLM, Ahmed AI, El-Hakim YMA (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: role of curcumin supplemented diet. Ecotoxicol Environ Saf 188:109890. https://doi.org/10.1016/j.ecoenv.2019.109890
Chen L, Zhang J, Zhu Y, Zhang Y (2018) Interaction of chromium(III) or chromium(VI) with catalase and its effect on the structure and function of catalase: an in vitro study. Food Chem 244:378–385. https://doi.org/10.1016/j.foodchem.2017.10.062
Marzec-Wróblewska U, Kamiński P, Łakota P, Szymański M, Wasilow K, Ludwikowski G, Jerzak L, Stuczyński T, Woźniak A, Buciński A (2019) Human sperm characteristics with regard to cobalt, chromium, and Lead in semen and activity of catalase in seminal plasma. Biol Trace Elem Res 188:251–260. https://doi.org/10.1007/s12011-018-1416-9
Acknowledgments
The authors are grateful to the Metal Speciation Lab at CSIR, National Institute of Oceanography, Dona Paula, Goa, India for the estimation of metals in the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Halder, S., Kar, R., Chakraborty, S. et al. Chromium Exposure in Late Gestation Period Caused Increased Levels of Cr in Brain Tissue: Association with Alteration of Activity and Gene Expression of Antioxidant Enzymes of F1 and F2 Generation Mice. Biol Trace Elem Res 199, 2635–2643 (2021). https://doi.org/10.1007/s12011-020-02367-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02367-6