Abstract
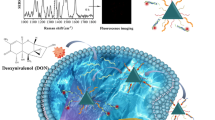
The fluorescent nanoprobes for reduced thiol compounds (represented by glutathione, GSH) are constructed based on the aggregation-induced emission (AIE) luminescence mechanism and endosome escape technology. First, a DNA sequence was designed with the decoration of biotin at the 5′-end, disulfide bound in the internal portion, and amino at the 3′-end. The aptamer of the MCF-7 cell was also one of the most important structures in our DNA sequence for the selectivity of MCF-7 cells. We modified streptavidin-modified magnetic beads (MB) with biotin-modified influenza virus hemagglutinin peptide (HA) and biotin-DNA-amino to form MB/DNA/HA. Carboxyl-modified tetraphenylethylene (TPE), an iconic AIE fluorogen, was bonded with amino-modified DNA by covalent interactions (TPE/DNA). Then, the TPE molecule was attached on the outer layer of MB via biotin-modified TPE/DNA to form MB/DNA/HA/TPE. Compared with traditional AIE/biomolecule conjugates, the nanoprobe had an enhanced endosome escape function, due to the assembly of HA. This construction made the intracellular fluorescence response more accurate. In the presence of reduced thiol compounds (take GSH, for example), the disulfide bond on the DNA was reduced by thiol-disulfide exchange reactions and the TPE molecule was released into the solution. The shedding TPE molecule was more hydrophobic than TPE/DNA and the conversion of TPE/DNA to shedding TPE could lead to the aggregation of the TPE fluorogen. Thus, its fluorescence was enhanced. Under the optimized condition, the fluorescence intensity increased with the increase in concentration of GSH‚ ranging from 1.0 × 10−9 M to 1.0 × 10−5 M‚ and the detection limit was 1.0 × 10−9 M. The relative standard deviation (RSD) was calculated to be 3.6%. The recovery in cell homogenate was from 94.5 to 102.7%. The nanoprobe provided a way for the detection of reduced thiol compounds in MCF-7 cells. We envision that, in the near future, our strategy of DNA-instructed AIE could be widely applied for biosensing and bioimaging in vitro and even in vivo with dramatically enhanced sensitivity.

Graphical Abstract





Similar content being viewed by others
References
Yin C, Xiong K, Huo F, Salamanca JC, Strongin RM. Fluorescent probes with multiple binding sites for the discrimination of Cys, Hcy, and GSH. Angew Chem Int Ed. 2017;56(43):13188–98.
Qin Y, Fan J, Yang W, Shen B, Yang Y, Zhou Q, et al. Endogenous Cys-assisted GSH@AgNCs-rGO nanoprobe for real-time monitoring of dynamic change in GSH levels regulated by natural drug. Anal Chem. 2020;92:1988–96.
Han X, Song X, Yu F, Chen L. A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress. Chem Sci. 2017;8:6991–7002.
Gong F, Cheng L, Yang N, Jin Q, Tian L, Wang M, et al. Bimetallic oxide MnMoOX nanorods for in vivo photoacoustic imaging of GSH and tumor-specific photothermal therapy. Nano Lett. 2018;18:6037–44.
Guo X, Wang L, Duval K, Fan J, Zhou S, Chen Z. Dimeric drug polymeric micelles with acid-active tumor targeting and FRET-traceable drug release. Adv Mater. 2018;30:1870020.
Yue Y, Huo F, Cheng F, Zhu X, Mafireyi T, Strongin RM, et al. Functional synthetic probes for selective targeting and multi-analyte detection and imaging. Chem Soc Rev. 2019;48:4155–77.
Song A, Feng T, Shen X, Gai S, Zhai Y, Chen H. Fluorescence detection of glutathione S-transferases in a low GSH level environment. Chem Commun. 2019;55:7219–22.
Xiong K, Huo F, Chao J, Zhang Y, Yin C. Colorimetric and NIR fluorescence probe with multiple binding sites for distinguishing detection of Cys/Hcy and GSH in vivo. Anal Chem. 2019;91(2):1472–8.
Mei J, Huang Y, Tian H. Progress and trends in AIE-based bioprobes: a brief overview. ACS Appl Mater Interfaces. 2018;10:12217–61.
Xia F, Wu J, Wu X, Hu Q, Dai J, Lou X. Modular design of peptide- or DNA-modified AIEgen probes for biosensing applications. Accounts Chem Res. 2019;52:3064–74.
Luo J, Xie Z, Lam JWY, Cheng L, Chen H, Qiu C, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun. 2001:1740–1.
Liu J, Su H, Meng L, Zhao Y, Deng C, Ng JCY, et al. What makes efficient circularly polarised luminescence in the condensed phase: aggregation-induced circular dichroism and light emission. Chem Sci. 2012;3(9):2737–47.
Qian J, Tang B. AIE luminogens for bioimaging and theranostics: from organelles to animals. Chem. 2017;3(1):56–91.
Gao Y, He Z, He X, Zhang H, Weng J, Yang X, et al. Dual-color emissive AIEgen for specific and label-free double-stranded DNA recognition and single-nucleotide polymorphisms detection. J Am Chem Soc. 2019;141:20097–106.
Wang X, Dai J, Min X, Yu Z, Cheng Y, Huang K, et al. DNA-conjugated amphiphilic aggregation-induced emission probe for cancer tissue imaging and prognosis analysis. Anal Chem. 2018;90:8162–9.
Min X, Zhuang Y, Zhang Z, Jia Y, Hakeem A, Zheng F, et al. Lab in a tube: sensitive detection of microRNAs in urine samples from bladder cancer patients using a single-label DNA probe with AIEgens. ACS Appl Mater Interfaces. 2015;7:16813–8.
Ding Y, Shi L, Wei H. A “turn on” fluorescent probe for heparin and its oversulfated chondroitin sulfate contaminant. Chem Sci. 2015;6:6361–6.
Sun F, Zhao S, Peng M, Fu Q, Gao H, Jia Y, et al. Sequencing of small DNA fragments with aggregated-induced-emission molecule-labeled nucleotides. Anal Chem. 2020. https://doi.org/10.1021/acs.analchem.0c00707.
Jing J, Xue Y, Liu Y, Xu B, Li H, Liu L, et al. Co-assembly of HPV capsid proteins and aggregation-induced emission fluorogens for improved cell imaging. Nanoscale. 2020;12:5501–6.
Chen X, Gao H, Deng Y, Jin Q, Ji J, Ding D. Supramolecular aggregation-induced emission nanodots with programmed tumor microenvironment responsiveness for image-guided orthotopic pancreatic cancer therapy. ACS Nano. 2020;14:5121–34.
Sun X, Ying N, Ju C, Li Z, Xu N, Qu G, et al. Modified beacon probe assisted dual signal amplification for visual detection of microRNA. Anal Biochem. 2018;550:68–71.
Guo Y, Wang H, Sun Y, Qu B. A disulfide bound-molecular beacon as a fluorescent probe for the detection of reduced glutathione and its application in cells. Chem Commun. 2012;48:3221–3.
Wi Y, Le H, Verwilst K, Sunwoo K, Kim S, Song J, et al. Modulating the GSH/Trx selectivity of a fluorogenic disulfide-based thiol sensor to reveal diminished GSH levels under ER stress. Chem Commun. 2018;54:8897–900.
Funding
This work was supported by the Development Plan of Youth Innovation Team in Colleges and Universities of Shandong Province (2020KJC003), the Natural Science Foundation of Shandong Province of China (ZR2016JL010), and the Primary Research and Development Plan of Shandong Province (2018GSF118172).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 738 kb)
Rights and permissions
About this article
Cite this article
Hu, Y., Cao, X., Guo, Y. et al. An aggregation-induced emission fluorogen/DNA probe carrying an endosome escaping pass for tracking reduced thiol compounds in cells. Anal Bioanal Chem 412, 7811–7817 (2020). https://doi.org/10.1007/s00216-020-02909-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02909-w