Abstract
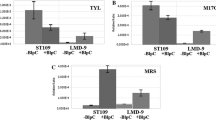
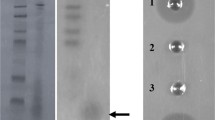
Bovicin HC5 is a peptide that has inhibitory activity against various pathogenic microorganisms and food spoilage bacteria. Aiming to improve the productivity of this bacteriocin, we evaluated several potential factors that could stimulate the synthesis of bovicin HC5 and selected variants of Streptococcus equinus (Streptococcus bovis) HC5 with enhanced bacteriocin production by adaptive laboratory evolution (ALE). The highest production of the bacteriocin (1.5-fold) was observed when Strep. equinus HC5 was cultivated with lactic acid (100 mmol/L). For the ALE experiment, Strep. equinus HC5 cells were subjected to acid-shock (pH 3.0 for 2 h) and maintained in continuous culture for approximately 140 generations (40 days) in media with lactic acid (100 mmol/L) and pH-controlled at 5.5 ± 0.2. An adapted variant was selected showing a distinct phenotype (sedimentation, pigmentation) compared with the parental strain. Bacteriocin production increased 2-fold in this adapted Strep. equinus HC5 variant, which appears to be associated with changes in the cell envelope of the adapted variant and enhanced bacteriocin release into the culture media. In addition, the adapted variant showed higher levels of expression of all bovicin HC5 biosynthetic genes compared with the parental strain during the early and late stages of growth. Results presented here indicate that ALE is a promising strategy for selecting strains of lactic acid bacteria with increased production of bacteriocins.





Similar content being viewed by others
References
Bagci U, Ozmen Togay S, Temiz A, Ay M (2019) Probiotic characteristics of bacteriocin-producing Enterococcus faecium strains isolated from human milk and colostrum. Folia Microbiol 64:735–750. https://doi.org/10.1007/s12223-019-00687-2
Castilho NPA, Colombo M, Oliveira LLd, Todorov SD, Nero LA (2019) Lactobacillus curvatus UFV-NPAC1 and other lactic acid bacteria isolated from calabresa, a fermented meat product, present high bacteriocinogenic activity against Listeria monocytogenes. BMC Microbiol 19:63. https://doi.org/10.1186/s12866-019-1436-4
Muhammad Z, Ramzan R, Abdelazez A, Amjad A, Afzaal M, Zhang S, Pan S (2019) Assessment of the antimicrobial potentiality and functionality of Lactobacillus plantarum strains isolated from the conventional inner mongolian fermented cheese against foodborne pathogens. Pathogens 8:71. https://doi.org/10.3390/pathogens8020071
Grosu-Tudor S-S, Stancu M-M, Pelinescu D, Zamfir M (2014) Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J Microbiol Biotechnol 30:2459–2469. https://doi.org/10.1007/s11274-014-1671-7
Perales-Adán J, Rubiño S, Martínez-Bueno M, Valdivia E, Montalbán-López M, Cebrián R, Maqueda M (2018) LAB bacteriocins controlling the food isolated (drug-resistant) Staphylococci. Front Microbiol 9:1143. https://doi.org/10.3389/fmicb.2018.01143
Todorov SD, Perin LM, Carneiro BM, Rahal P, Holzapfel W, Nero LA (2017) Safety of Lactobacillus plantarum ST8Sh and its bacteriocin. Probiotics Antimicrob Proteins 9:334–344. https://doi.org/10.1007/s12602-017-9260-3
Eijsink VGH, Axelsson L, Diep DB, Håvarstein LS, Holo H, Nes IF (2002) Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 81:639–654. https://doi.org/10.1023/A:1020582211262
Riley MA, Gordon DM (1999) The ecological role of bacteriocins in bacterial competition. Trends Microbiol 7:129–133. https://doi.org/10.1016/S0966-842X(99)01459-6
Li D, Ni K, Pang H, Wang Y, Cai Y, Jin Q (2015) Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australas J Anim Sci 28:620–631. https://doi.org/10.5713/ajas.14.0439
Russell JB, Mantovani HC (2002) The bacteriocins of ruminal bacteria and their potential as an alternative to antibiotics. J Mol Microbiol Biotechnol 4:347–355
Mantovani HC, Hu H, Worobo RW, Russell JB (2002) Bovicin HC5, a bacteriocin from Streptococcus bovis HC5. Microbiology 148:3347–3352. https://doi.org/10.1099/00221287-148-11-3347
Mantovani HC, Russell JB (2001) Nisin resistance of Streptococcus bovis. Appl Environ Microbiol 67:808–813. https://doi.org/10.1128/AEM.67.2.808-813.2001
Azevedo AC, Bento CBP, Ruiz JC, Queiroz MV, Mantovani HC (2015) Draft genome sequence of Streptococcus equinus (Streptococcus bovis) HC5, a lantibiotic producer from the bovine rumen. Genome Announc 3:e00085–e00015. https://doi.org/10.1128/genomeA.00085-15
Houlihan AJ, Mantovani HC, Russell JB (2004) Effect of pH on the activity of bovicin HC5, a bacteriocin from Streptococcus bovis HC5. FEMS Microbiol Lett 231:27–32. https://doi.org/10.1016/S0378-1097(03)00922-4
Paiva AD, Fernandes KM, Dias RS, dos Santos RA, Licursi de Oliveira L, Neves CA, Oliveira de Paula S, Mantovani HC (2013) Safety evaluation of the antimicrobial peptide bovicin HC5 orally administered to a murine model. BMC Microbiol 13:69. https://doi.org/10.1186/1471-2180-13-69
Wescombe PA, Tagg JR (2003) Purification and characterization of streptin, a type A1 lantibiotic produced by Streptococcus pyogenes. Appl Environ Microbiol 69:2737–2747. https://doi.org/10.1128/aem.69.5.2737-2747.2003
Barbosa AAT, Mantovani HC, Lopes DRG, Santana HF (2015) Like it acid and poor: a study of abiotic factors influencing Streptococcus bovis HC5 growth and bacteriocin production. J Microbiol Biotechnol Food Sci 4:421–426. https://doi.org/10.15414/jmbfs.2015.4.5.421-426
Carvalho AAT, Mantovani HC, Paiva AD, de Melo MR (2009) The effect of carbon and nitrogen sources on bovicin HC5 production by Streptococcus bovis HC5. J Appl Microbiol 107:339–347. https://doi.org/10.1111/j.1365-2672.2009.04212.x
Jiang Y, Ren F, Liu S, Zhao L, Guo H, Hou C (2016) Enhanced acid tolerance in Bifidobacterium longum by adaptive evolution: comparison of the genes between the acid-resistant variant and wild-type strain. J Microbiol Biotechnol 26:452–460. https://doi.org/10.4014/jmb.1508.08030
Ju SY, Kim JH, Lee PC (2016) Long-term adaptive evolution of Leuconostoc mesenteroides for enhancement of lactic acid tolerance and production. Biotechnol Biofuels 9:240. https://doi.org/10.1186/s13068-016-0662-3
Reyes LH, Almario MP, Winkler J, Orozco MM, Kao KC (2012) Visualizing evolution in real time to determine the molecular mechanisms of n-butanol tolerance in Escherichia coli. Metab Eng 14:579–590. https://doi.org/10.1016/j.ymben.2012.05.002
Warsi OM, Andersson DI, Dykhuizen DE (2018) Different adaptive strategies in E. coli populations evolving under macronutrient limitation and metal ion limitation. BMC Evol Biol 18:72. https://doi.org/10.1186/s12862-018-1191-4
Zhang J, Wu C, Du G, Chen J (2012) Enhanced acid tolerance in Lactobacillus casei by adaptive evolution and compared stress response during acid stress. Biotechnol Bioprocess Eng 17:283–289. https://doi.org/10.1007/s12257-011-0346-6
Mantovani HC, Russell JB (2003b) Factors affecting the antibacterial activity of the ruminal bacterium, Streptococcus bovis HC5. Curr Microbiol 46:0018–0023. https://doi.org/10.1007/s00284-002-3786-6
Nagaraja TG, Titgemeyer EC (2007) Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook 1, 2. J Dairy Sci 90:E17–E38. https://doi.org/10.3168/jds.2006-478
Chang JY, Lee HJ, Chang HC (2007) Identification of the agent from Lactobacillus plantarum KFRI464 that enhances bacteriocin production by Leuconostoc citreum GJ7. J Appl Microbiol 103:2504–2515. https://doi.org/10.1111/j.1365-2672.2007.03543.x
Valadares Filho SC, Costa e Silva LF, Lopes SA, Prados LF, Chizzotti ML, Machado PAS, Bissaro LZ, Furtado T (2016) BR-CORTE 3.0. Nutritional requirements, diet formulation and performance prediction of Zebu and Crossbred cattle. Available in: www.brcorte.com.br. Accessed in: 12/11/2018
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394. https://doi.org/10.1146/annurev.mi.03.100149.002103
Houlihan AJ, Russell JB (2006) Factors affecting the activity of bovicin HC5, a bacteriocin from Streptococcus bovis HC5: release, stability and binding to target bacteria. J Appl Microbiol 100:168–174. https://doi.org/10.1111/j.1365-2672.2005.02745.x
Hoover DG, Harlander SK (1993) Chapter 2 - screening methods for detecting bacteriocin activity. In: Bacteriocins of lactic acid bacteria. Academic Press, pp. 23–39. doi: https://doi.org/10.1016/B978-0-12-355510-6.50010-5
Joseph B, Dhas B, Hena V, Raj J (2013) Bacteriocin from Bacillus subtilis as a novel drug against diabetic foot ulcer bacterial pathogens. Asian Pac J Trop Biomed 3:942–946. https://doi.org/10.1016/S2221-1691(13)60183-5
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI, Inc, Newark, DE, USA
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33. https://doi.org/10.1111/j.1574-6968.1980.tb05599.x
Souza GB, Mendes TAO, Fontes PP, Barros VA, Gonçalves AB, Ferreira TF, Costa MD-BL, Alves MS, Fietto LG (2019) Genome-wide identification and expression analysis of dormancy-associated gene 1/auxin repressed protein (DRM1/ARP) gene family in Glycine max. Prog Biophys Mol Biol 146:134–141. https://doi.org/10.1016/j.pbiomolbio.2019.03.006
Hernández J, Benedito JL, Abuelo A, Castillo C (2014) Ruminal acidosis in feedlot: from aetiology to prevention. Sci World J 2014:702572–702572. https://doi.org/10.1155/2014/702572
Nilsson L, Nielsen MK, Ng Y, Gram L (2002) Role of acetate in production of an autoinducible class IIa bacteriocin in Carnobacterium piscicola A9b. Appl Environ Microbiol 68:2251–2260. https://doi.org/10.1128/AEM.68.5.2251-2260.2002
Dallinger WH (1887) The President’s address. J R Microsc Soc 7:185–199. https://doi.org/10.1111/j.1365-2818.1887.tb01566.x
Hass JW (2000) The Reverend Dr William Henry Dallinger, F.R.S. (1839-1909). Notes Rec R Soc Lond 54:53–65. https://doi.org/10.1098/rsnr.2000.0096
Kwon YW, Bae J-H, Kim S-A, Han NS (2018) Development of freeze-thaw tolerant Lactobacillus rhamnosus GG by adaptive laboratory evolution. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02781
Taweecheep P, Naloka K, Matsutani M, Yakushi T, Matsushita K, Theeragool G (2019) In vitro thermal and ethanol adaptations to improve vinegar fermentation at high temperature of Komagataeibacter oboediens MSKU 3. Appl Biochem Biotechnol 189:144–159. https://doi.org/10.1007/s12010-019-03003-3
López-González MJ, Escobedo S, Rodríguez A, Neves AR, Janzen T, Martínez B (2018) Adaptive evolution of industrial Lactococcus lactis under cell envelope stress provides phenotypic diversity. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02654
Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Medina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515. https://doi.org/10.1016/S0734-9750(02)00050-2
Warnecke T, Gill RT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Factories 4:25–25. https://doi.org/10.1186/1475-2859-4-25
Lindahl T, Nyberg B (1972) Rate of depurination of native deoxyribonucleic acid. Biochem 11:3610–3618. https://doi.org/10.1021/bi00769a018
Cotter PD, Hill C (2003) Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453. https://doi.org/10.1128/mmbr.67.3.429-453.2003
Foster PL (2007) Stress-induced mutagenesis in bacteria. Crit Rev Biochem Mol Biol 42:373–397. https://doi.org/10.1080/10409230701648494
Liu S, Ren F, Jiang J, Zhao L (2016) Acid response of Bifidobacterium longum subsp. longum BBMN68 is accompanied by modification of the cell membrane fatty acid composition. J Microbiol Biotechnol 26:1190–1197. https://doi.org/10.4014/jmb.1511.11013
Wu C, Zhang J, Wang M, Du G, Chen J (2012) Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J Ind Microbiol Biotechnol 39:1031–1039. https://doi.org/10.1007/s10295-012-1104-2
Yang X, Hang X, Zhang M, Liu X, Yang H (2015) Relationship between acid tolerance and cell membrane in Bifidobacterium, revealed by comparative analysis of acid-resistant derivatives and their parental strains grown in medium with and without Tween 80. Appl Microbiol Biotechnol 99:5227–5236. https://doi.org/10.1007/s00253-015-6447-y
Baker JL, Abranches J, Faustoferri RC, Hubbard CJ, Lemos JA, Courtney MA, Quivey R Jr (2015) Transcriptional profile of glucose-shocked and acid-adapted strains of Streptococcus mutans. Mol Oral Microbiol 30:496–517. https://doi.org/10.1111/omi.12110
Wu C-w, Spike T, Klingeman DM, Rodriguez M, Bremer VR, Brown SD (2017) Generation and characterization of acid tolerant Fibrobacter succinogenes S85. Sci Rep 7:2277. https://doi.org/10.1038/s41598-017-02628-w
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brasília, Brazil, Grant #432920/2018-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brasília, Brazil, Grant #0001), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; Belo Horizonte, Brazil), and INCT Ciência Animal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed following a protocol approved by the Ethics and Production Animal Care and Use Committee of the Universidade Federal de Viçosa (CEUAP-UFV, Protocol 01/2019).
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 289 kb)
Rights and permissions
About this article
Cite this article
Moreira, S.M., de Oliveira Mendes, T.A. & Mantovani, H.C. Stimulation of Bovicin HC5 Production and Selection of Improved Bacteriocin-Producing Streptococcus equinus HC5 Variants. Probiotics & Antimicro. Prot. 13, 899–913 (2021). https://doi.org/10.1007/s12602-020-09703-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09703-1