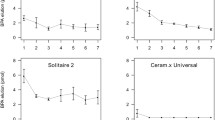
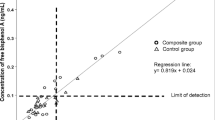
Abstract
Bisphenol A and phthalates are endocrine disruptors widely used as chemical additives mainly in plastic products, including materials for dentistry procedures. Besides, many plasticizers have been associated with important diseases requiring performed methods for their quantification. In the present study, an alternative method for the determination of bisphenol A (BPA) and phthalate metabolites in saliva was developed and validated using hollow fiber liquid phase microextraction (HF-LPME) for sample preparation and gas chromatography coupled to ion trap mass spectrometry (GC/MS) for analysis. A mixture of octanol and ethyl octanoate (1:1 v/v) was used as an acceptor phase in hollow fiber to extract the analytes from saliva samples. A Doehlert design was performed to optimize the variable sample agitation and extraction time. The HF-LPME-GC/MS method developed for saliva analysis showed good selectivity, linearity (R2 > 0.900), and precision (CV = 0.86–18.68%). Limits of detection and quantification ranged from 0.03 to 0.53 μg L−1 and 0.09 to 1.78 μg L−1, respectively. A high concentration of BPA in the oral cavity and oropharyngeal space is a warning of the possible association with the main cancer of the mouth. The method developed and validated was applied to patients with oral squamous cell carcinoma (study group, n = 16) and patients who did not present any oral lesion (control group, n = 16). A principal component analysis was performed and showed a tendency for the association between oral squamous cell carcinoma (OSCC) and plasticizers.

Graphical abstract




Similar content being viewed by others
References
North EJ, Halden RU. Plastics and environmental health: the road ahead. Rev Environ Health. 2013;28:1–8. https://doi.org/10.1515/reveh-2012-0030.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017. https://doi.org/10.1126/sciadv.1700782.
Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–110. https://doi.org/10.1210/en.2012-1422.
Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–94. https://doi.org/10.1146/annurev.publhealth.012809.103714.
Hwang KA, Park MA, Kang NH, Yi BR, Hyun SH, Jeung EB, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 β-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013;272:637–46. https://doi.org/10.1016/j.taap.2013.07.027.
Wetherill YB, Fisher NL, Staubach A, Danielsen M, De Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005;65:54–65.
Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. https://doi.org/10.1016/j.tox.2006.06.009.
Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–40. https://doi.org/10.1016/j.fct.2012.07.059.
Geens T, Goeyens L, Covaci A. Are potential sources for human exposure to bisphenol-A overlooked? Int J Hyg Environ Health. 2011;214:339–47. https://doi.org/10.1016/j.ijheh.2011.04.005.
Altamirano GA, Muñoz-de-Toro M, Luque EH, Gómez AL, Delconte MB, Kass L. Milk lipid composition is modified by perinatal exposure to bisphenol A. Mol Cell Endocrinol. 2015;411:258–67. https://doi.org/10.1016/j.mce.2015.05.007.
Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36:699–703. https://doi.org/10.1016/j.placenta.2015.04.002.
Kanuga S. Bisphenol A (BPA) may be released in the oral cavity after sealant placement. J Am Dent Assoc. 2014;145:1272–3. https://doi.org/10.14219/jada.2014.79.
Kingman A, Hyman J, Masten SA, Jayaram B, Smith C, Eichmiller F, et al. Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J Am Dent Assoc. 2012;143:1292–302. https://doi.org/10.14219/jada.archive.2012.0090.
Russo G, Barbato F, Mita DG, Grumetto L. Simultaneous determination of fifteen multiclass organic pollutants in human saliva and serum by liquid chromatography–tandem ultraviolet/fluorescence detection: a validated method. Biomed Chromatogr. 2019. https://doi.org/10.1002/bmc.4427.
Mallozzi M, Leone C, Manurita F, Bellati F, Caserta D. Endocrine disrupting chemicals and endometrial cancer: an overview of recent laboratory evidence and epidemiological studies. Int J Environ Res Public Health. 2017. https://doi.org/10.3390/ijerph14030334.
Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90. https://doi.org/10.1210/jc.2002-020209.
Shafei A, Ramzy MM, Hegazy AI, Husseny AK, EL-hadary UG, Taha MM, et al. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene. 2018;647:235–43. https://doi.org/10.1016/j.gene.2018.01.016.
Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho SM. Exposure to bisphenol a correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0090332.
Emfietzoglou R, Spyrou N, Mantzoros CS, Dalamaga M. Could the endocrine disruptor bisphenol-A be implicated in the pathogenesis of oral and oropharyngeal cancer? Metabolic considerations and future directions. Metabolism. 2019;91:61–9. https://doi.org/10.1016/j.metabol.2018.11.007.
Murata M, Kang JH. Bisphenol A (BPA) and cell signaling pathways. Biotechnol Adv. 2018;36:311–27. https://doi.org/10.1016/j.biotechadv.2017.12.002.
Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019. https://doi.org/10.1016/j.envint.2019.04.040.
Li R, Xing Q, Wu X, Zhang L, Tang M, Tang J, et al. Di-n-butyl phthalate epigenetically induces reproductive toxicity via the PTEN/AKT pathway. Cell Death Dis. 2019. https://doi.org/10.1038/s41419-019-1547-8.
Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118:1039–46. https://doi.org/10.1289/ehp.0901690.
Tranfo G, Caporossi L, Paci E, Aragona C, Romanzi D, De Carolis C, et al. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicol Lett. 2012;213:15–20. https://doi.org/10.1016/j.toxlet.2011.11.033.
Bin Huang H, Pan WH, Chang JW, Chiang HC, Guo YL, Jaakkola JJK, et al. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ Res. 2017;153:63–72. https://doi.org/10.1016/j.envres.2016.11.014.
Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep. 2015;2:379–87. https://doi.org/10.1007/s40572-015-0066-8.
Agas D, Sabbieti MG, Capacchietti M, Materazzi S, Menghi G, Materazzi G, et al. Benzyl butyl phthalate influences actin distribution and cell proliferation in rat Py1a osteoblasts. J Cell Biochem. 2007;101:543–51. https://doi.org/10.1002/jcb.21212.
Lee HR, Hwang KA, Choi KC. The estrogen receptor signaling pathway activated by phthalates is linked with transforming growth factor-β in the progression of LNCaP prostate cancer models. Int J Oncol. 2014;45:595–602. https://doi.org/10.3892/ijo.2014.2460.
Zhu M, Huang C, Ma X, Wu R, Zhu W, Li X, et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ Toxicol Pharmacol. 2018;63:29–33. https://doi.org/10.1016/j.etap.2018.08.007.
Zuccarello P, Conti GO, Cavallaro F, Copat C, Cristaldi A, Fiore M, et al. Implication of dietary phthalates in breast cancer. A systematic review. Food Chem Toxicol. 2018;118:667–74. https://doi.org/10.1016/j.fct.2018.06.011.
Fernandez MAM, André LC, Cardeal ZL. Hollow fiber liquid-phase microextraction-gas chromatography-mass spectrometry method to analyze bisphenol A and other plasticizer metabolites. J Chromatogr A. 2017;1481:31–6. https://doi.org/10.1016/j.chroma.2016.12.043.
Akgül S, Sur Ü, Düzçeker Y, Balcı A, Kızılkan MP, Kanbur N, et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(12):1084–7. https://doi.org/10.1080/09513590.2019.1630608.
Liu C, Deng YL, Zheng TZ, Yang P, Jiang XQ, Liu EN, et al. Urinary biomarkers of phthalates exposure and risks of thyroid cancer and benign nodule. J Hazard Mater. 2020. https://doi.org/10.1016/j.jhazmat.2019.121189.
Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health. 2011;119:1396–402. https://doi.org/10.1289/ehp.1103582.
Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon EY, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015;36:S232–53. https://doi.org/10.1093/carcin/bgv038.
Del Pup L, Mantovani A, Luce A, Cavaliere C, Facchini G, Di Francia R, et al. Endocrine disruptors and female cancer: informing the patients. Oncol Rep. 2015;34:302–10. https://doi.org/10.3892/or.2015.3997.
Tsochatzis ED, Tzimou-Tsitouridou R, Gika HG. Analytical methodologies for the assessment of phthalate exposure in humans. Crit Rev Anal Chem. 2017;47:279–97. https://doi.org/10.1080/10408347.2016.1273754.
Kumar AR, Sivaperumal P. Analytical methods for the determination of biomarkers of exposure to phthalates in human urine samples. TrAC Trends Anal Chem. 2016;75:151–61. https://doi.org/10.1016/j.trac.2015.06.008.
Kokosa JM. Selecting an extraction solvent for a greener liquid phase microextraction (LPME) mode-based analytical method. TrAC Trends Anal Chem. 2019;118:238–47. https://doi.org/10.1016/j.trac.2019.05.012.
Reyes-Garcés N, Gionfriddo E. Recent developments and applications of solid phase microextraction as a sample preparation approach for mass-spectrometry-based metabolomics and lipidomics. TrAC Trends Anal Chem. 2019;113:172–81. https://doi.org/10.1016/j.trac.2019.01.009.
Moura HSRP, Rocha PRS, Amato AA, Sodré FF. Quantification of bisphenol A in urine samples from children studying in public schools from the Brazilian Capital. Microchem J. 2020;152. https://doi.org/10.1016/j.microc.2019.104347.
Fisher M, Arbuckle TE, MacPherson S, Braun JM, Feeley M, Gaudreau É. Phthalate and BPA exposure in women and newborns through personal care product use and food packaging. Environ Sci Technol. 2019;53:10813–26. https://doi.org/10.1021/acs.est.9b02372.
Viñas P, Campillo N, Martínez-Castillo N, Hernández-Córdoba M. Comparison of two derivatization-based methods for solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal Bioanal Chem. 2010;397:115–25. https://doi.org/10.1007/s00216-010-3464-7.
Amin MM, Ebrahim K, Poursafa P. Development of a dispersive liquid–liquid microextraction (DLLME) method coupled with GC/MS as a simple and valid method for simultaneous determination of phthalate metabolites in plasma. Int J Environ Anal Chem. 2017;97:1362–77. https://doi.org/10.1080/03067319.2017.1422497.
Venson R, Korb AS, Cooper G. A review of the application of hollow-fiber liquid-phase microextraction in bioanalytical methods – a systematic approach with focus on forensic toxicology. J Chromatogr B. 2019;1108:32–53. https://doi.org/10.1016/j.jchromb.2019.01.006.
Pedersen-Bjergaard S, Rasmussen KE. Liquid-phase microextraction with porous hollow fibers, a miniaturized and highly flexible format for liquid-liquid extraction. J Chromatogr A. 2008;1184:132–42.
Cousins IT, Mackay D, Parkerton TF. Physical-chemical properties and evaluative fate modelling of phthalate esters. Handb Environ Chem. 2003;3:57–84. https://doi.org/10.1007/b11463.
The Metabolomics Innovation Center: Human metabolom database. 2020. https://hmdb.ca/metabolites. Accessed 10 Ago 2020.
Royal Society of Chemistry: Chemspider-Search and share chemistry. http://www.chemspider.com/Default.aspx. Accessed 10 Ago 2020.
Silva MJ, Reidy JA, Samandar E, Herbert AR, Needham LL, Calafat AM. Detection of phthalate metabolites in human saliva. Arch Toxicol. 2005;79:647–52. https://doi.org/10.1007/s00204-005-0674-4.
Magnusson B, Örnemark U. Eurachem guide: the fitness for purpose of analytical methods – a laboratory guide to method validation and related topics. 2nd ed. 2014. ISBN 978-91-87461-59-0. https://www.eurachem.org/index.php/publications/guides/mv. Accessed 17 Jul 2020.
Fernández MA, Gómara B, González MJ. Occurrence of phthalates and their metabolites in the environment and human health implications. In: Barceló D, editor. Emerging organic contaminants and human health. The Handbook of Environmental Chemistry. Berlin, Heidelberg; 2012. https://doi.org/10.1007/698_2011_127.
Campaña AMG, Rodrigues LC, González AL, Barreto FA, Vidal JLM. Sequential response surface methodology for multioptimization in analytical chemistry with three-variable Doehlert design. Anal Chim Acta. 1997;348:237–46. https://doi.org/10.1016/S0003-2670(97)00155-4.
Van Der Meer TP, Van Faassen M, Frederiksen H, Van Beek AP, Wolffenbuttel BHR, Kema IP, et al. Development and interlaboratory validation of two fast UPLC-MS-MS methods determining urinary bisphenols, parabens and phthalates. J Anal Toxicol. 2019;43:452–64. https://doi.org/10.1093/jat/bkz027.
Herrero L, Calvarro S, Fernández MA, Quintanilla-López JE, González MJ, Gómara B. Feasibility of ultra-high performance liquid and gas chromatography coupled to mass spectrometry for accurate determination of primary and secondary phthalate metabolites in urine samples. Anal Chim Acta. 2015;853:625–36. https://doi.org/10.1016/j.aca.2014.09.043.
Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26:803–24. https://doi.org/10.1111/j.1539-6924.2006.00770.x.
Domínguez-Romero E, Scheringer M. A review of phthalate pharmacokinetics in human and rat: what factors drive phthalate distribution and partitioning? Drug Metab Rev. 2019;51:314–29. https://doi.org/10.1080/03602532.2019.1620762.
Bouma K, Schakel DJ. Migration of phthalates from PVC toys into saliva simulant by dynamic extraction. Food Addit Contam. 2002;19:602–10. https://doi.org/10.1080/02652030210125137.
Funding
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Funding was also provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) and Ministério da Saúde do Brasil.
Author information
Authors and Affiliations
Contributions
José Messias Gomes: conceptualization, methodology, investigation, validation, writing—original draft. Tatiana Fernandes Araujo Almeida: writing—review and editing, methodology. Tarcília Aparecida da Silva: writing—review and editing, resources: Zenilda de Lourdes Cardeal: data curation, project administration, resources. Helvécio Costa Menezes: software, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Dedicated to the memory of Gabriela Dias Cerqueira, who developed the method and sadly passed away prematurely.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, J.M., Almeida, T.F.A., da Silva, T.A. et al. Saliva biomonitoring using LPME-GC/MS method to assess dentistry exposure to plasticizers. Anal Bioanal Chem 412, 7799–7810 (2020). https://doi.org/10.1007/s00216-020-02908-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02908-x