Abstract
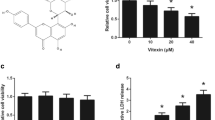
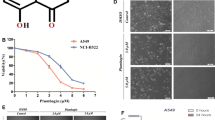
Myricetin is categorized under the secondary metabolite flavonoid which includes a diverse range of consumable plant parts, and it has a potential against several classes of cancer including cancers and tumors. In the present study, the anticancer potential of the unique flavonoid-myricetin in A549 lung cancer cells was evaluated. Among different doses of myricetin, 73 μg/ml was more effective to prevent the cancer cell growth. It also promoted sub-G1 phase aggregation of cells and a equivalent decrease in the fraction of cells entering the S and subsequent phase which indicates apoptotic cell death. Myricetin generated enormous free radicals and, altered the potential of mitochondrial membrane in A549 cells as paralleled to untreated cells. In addition, myricetin treatment intensified the expression of P53 and relegated the expression of EGFR in A549 cells. These results suggested that myricetin exhibits cytotoxic potential by arresting the progression of cell cycle and ROS–dependent mitochondria-mediated mortality in cancer A549 lung cancer cells and it would be useful to develop as a drug candidate for lung cancer therapeutics. In silico experiments were carried out against human EGFR and P53 tumor suppressor protein to gain more insights into the binding mode of the myricetin may act as significant potential for anticancer therapy.












Similar content being viewed by others
References
Ochwangi DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, Kiama SG (2014) Medicinal plants used in treatment and management of cancer in Kakamega County Kenya. J Ethnopharmacol 151:1040–1055
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Barabadi H, Vahidi H, Damavandi Kamali K, Rashedi M, Hosseini O, Golnaraghi Ghomi AR, Saravanan M (2019) Emerging theranostic silver nanomaterials to combat colorectal cancer: a systematic review. J Clust Sci 31:311–321
Armania N, Yazan LS, Ismail IS, Foo JB, Tor YS, Ishak N, Ismail N, Ismail M (2013) Dillenia Suffruticosa extract inhibits proliferation of human breast cancer cell lines (MCF-7 and MDA-MB-231) via induction of G2/M arrest and apoptosis. Molecules 18:13320–13339
Aszalos A (2008) Role of ATP-binding cassette (ABC) transporters in interactions between natural products and drugs. Curr Drug Metab 9:1010–1018
Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD (2009) Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 89:905–912
Chahar MK, Sharma N, Dobhal MP, Joshi YC (2011) Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 5:1–12
Semwal DK, Semwal RB, Combrinck S, Viljoen A (2016) Myricetin: a dietary molecule with diverse biological activities. Nutrients 8:1–31
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 2:19–34
Miean KH, Mohamed S (2001) Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 49:3106–3112
Cushnie T, Lamb A (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agent 26:343–356
Li Y, Ding Y (2012) Minireview: therapeutic potential of myricetin in diabetes mellitus. Food Sci Hum Wellnes 1:19–25
Rahimi R, Mahdavi M, Pejman S, Zare P, Balalaei S (2015) Inhibition of cell proliferation and induction of apoptosis in K562 human leukemia cells by the derivative (3-NpC) from dihydro-pyranochromenes family. Acta Biochim Pol 62:3–88
Nunez R (2001) DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol 3:67–70
Lakshmi S, Dhanaya GS, Joy B, Beena J, Padmaja G, Remani P (2008) Inhibitory effect of an extract of Curcuma zedoariae on human cervical carcinoma cells. J Med Chem 17:335–344
Premnath D, Enoch IV, Selvakumar PM, Indiraleka M, Vennila JJ (2017) Design, synthesis, spectral analysis, in vitro anticancer evaluation and molecular docking studies of some fluorescent 4-amino-2, 3-dimethyl-1-phenyl-3-pyrazolin-5-one, ampyrone derivatives. Interdisc Sci 9(1):130–139
Premnath D, Selvakumar PM, Ravichandiran P, Selvan GT, Indiraleka M, Vennila JJ (2016) Synthesis and spectroscopic characterization of fluorescent 4-aminoantipyrine analogues: molecular docking and in vitro cytotoxicity studies. Spectrochim Acta Part A 153:118–123
Moyo B, Mukanganyama S (2015) Antiproliferative activity of T. welwitschii extract on jurkat T cells. In Vitro BioMed Res Int 817624:1–10
Pajaniradje S, Mohankumar K, Pamidimukkala R, Subramanian S, Rajagopalan R (2014) Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. Biomed Res Int 474953:1–11
Priya B, Anil KS (2013) Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 3:439–459
Ha TK, Jung I, Kim ME, Bae SK, Lee JS (2017) Anti-cancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction–mediated apoptosis. Biomed Pharmacother 91:378–384
Sun W, Tao YX, Yu D, Zhao T, Wu L, Yu W, Han W (2018) Myricetin exerts potent anticancer effects on human skin tumor cells. Trop J Pharm Res 17:1067–1072
Xu N, Fang W, Mu L, Tang Y, Gao L, Ren S et al (2016) Overexpression of wildtype EGFR is tumorigenic and denotes a therapeutic target in non-small cell lung cancer. Oncotarget 7:3884–3896
Sun F, Zheng XY, Ye J, Wu TT, Wang J, Chen W (2012) Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer 64:599–606
Ye C, Zhang C, Huang H, Yang B, Xiao G, Kong D et al (2018) The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting PIM1 and disrupting the PIM1/ CXCR4 interaction. Cell Physiol Biochem 48:1230–1244
Duyumus HG, Ciftci GA, Yildirim SU, Demirci B, Kirimer N (2014) The cytotoxic activity of Vitex agnus castus L. essential oils and their biochemical mechanisms. Ind Crops Prod 55:33–42
Nagappan A, Lee HJ, Saralamma VV, Park HS, Hong GE, Yumnam S et al (2015) Flavonoids isolated from Citrus platymamma induced G2/M cell cycle arrest and apoptosis in A549 human lung cancer cells. Oncol Lett 12:1394–1402
Vermes I, Haanen C, Reutelingsperger C (2000) Flow cytometry of apoptotic cell death. J Immunol Methods 243:167–190
Tsui KC, Chiang TH, Wang JS, Lin LJ, Chao WC, Chen BH, Lu JF (2014) Flavonoids from Gynostemma pentaphyllum exhibit differential induction of cell cycle arrest in H460 and A549 cancer cells. Molecules 19:17663–17681
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28:9–21
Thamizhiniyan V, Young-Woong C, Young-Kyoon K (2015) The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J Biol Sci 22:752–759
Kuete V, Fokou FW, Karaosmanoglu O, Beng VP, Sivas H (2017) Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complement Altern Med 17:1–9
Wu X, Zhu H, Yan J, Khan M, Yu X (2017) Santamarine inhibits NF-κB activation and induces mitochondrial apoptosis in A549 lung adenocarcinoma cells via oxidative stress. Biomed Res Int 4734127:1–11
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 3164734:1–23
Rao PC, Begum S, Sahai M, Sriram DS (2017) Coptisine-induced cell cycle arrest at G2/M phase and reactive oxygen species–dependent mitochondria-mediated apoptosis in non-small-cell lung cancer A549 cells. Tumor Biol 39:1–13
Xu Y, Xie Q, Wu S, Yi D, Yu Y, Liu S, Li S, Li Z (2016) Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double strand breaks in human ovarian cancer cells. Mol Med Rep 13:2094–2100
Zhang L, Zhang J, Hu C, Cao J, Zhou X, Hu Y, He Q, Yang B (2009) Efficient activation of p53 pathway in A549 cells exposed to L2, a novel compound targeting p53–MDM2 interaction. Anti-Cancer Drug 20:416–424
Singh P, Bast F (2015) Screening and biological evaluation of myricetin as a multiple target inhibitor insulin, epidermal growth factor, and androgen receptor; in silico and in vitro. Investig New Drugs 33(3):575–593
Vinutha K, Pavan G, Pattar S, Kumari NS, Vidya SM (2019) Aqueous extract from Madhuca indica bark protects cells from oxidative stress caused by electron beam radiation: in vitro, in vivo and in silico approach. Heliyon 5(5):e01749
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajendran, P., Maheshwari, U., Muthukrishnan, A. et al. Myricetin: versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS–reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol Cell Biochem 476, 57–68 (2021). https://doi.org/10.1007/s11010-020-03885-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03885-6