Abstract
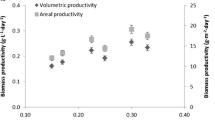
In this work, the previously proposed Fibonacci-type photobioreactor is scaled up and evaluated to produce Dunaliella salina. First, the composition of the culture medium was optimized to achieve maximal productivity. Next, the Fibonacci-type reactor was scaled up to 1250 L maintaining high solar radiation interception capacity of this type of reactor. Finally, the performance of the reactor for the production of green cells of Dunaliella salina at the environmental conditions prevailing in the Atacama Desert was evaluated. Data demonstrated that the proposed photobioreactor allows the temperature, pH and dissolved oxygen concentration to be maintained within the optimal ranges recommended for the selected strain. Both better exposure to solar radiation and photonic flow dilution avoids the use of cooling systems to prevent overheating under outdoor conditions. The system allows up to 60% more solar radiation to be intercepted than does the horizontal surface, likewise, allowing to maintain the pH efficiently through CO2 injection and to keep the dissolved oxygen concentration in acceptable ranges, thanks to its adequate mass transfer capacity. The biomass concentration reached up to 0.96 g L−1, three times higher than that obtained in a raceway reactor under the same environmental conditions, whereas productivity was up to 0.12 g L−1 day (2.41 g m−2 day). Maximum specific outdoor growth rates reached up to 0.17 day−1. Undoubtedly, this technology scaled up constitutes a new type of photobioreactor for use at the industrial scale since it is capable of maximizing biomass productivity under high light conditions.









Similar content being viewed by others
References
Sigamani, S., Ramamurthy, D., & Natarajan, H. (2016). A review on potential biotechnological applications of microalgae. Journal of Applied Pharmaceutical Science, 6(8), 179–184. https://doi.org/10.7324/JAPS.2016.60829.
Koller, M., Muhr, A., & Braunegg, G. (2014). Microalgae as versatile cellular factories for valued products. Algal Research, 6(PA), 52–63. https://doi.org/10.1016/j.algal.2014.09.002.
Mobin, S., & Alam, F. (2017). Some promising microalgal species for commercial applications: a review. In Energy Procedia (Vol. 110). https://doi.org/10.1016/j.egypro.2017.03.177
Ramana, K. V., Xavier, J. R., & Sharma, R. K. (2017). Recent trends in pharmaceutical biotechnology. Pharmaceutical Biotechnology: Current Research, 1(1:5), 1–10.
Ben-Amotz, A. (1995). New mode of Dunaliella biotechnology: two-phases for ß-carotene production. Applied Phycology, 7, 65–68.
G.M.I. (2015). Beta carotene market size, industry analysis report, regional outlook, application development totential, price trend, competitive market share & forecast, 2020 - 2026. Global Market Insigts.
Demirbas, A., & Demirba, A. (2010). Use of algae as biofuel sources. Energy Conversion and Management, 51(12), 2738–2749.
Ben-Amotz, A. (1995). New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. Journal of Applied Phycology, 7(1), 65–68. https://doi.org/10.1007/BF00003552.
Hamed, I., AK, B., Isik, O., Uslu, L., & Vursavus, K. K. (2017). The effect of temperature and salinity on the growth and carotenogenesis of three Dunaliella species (Dunaliella sp. Lake Isolate, Dunaliella salina CCAP 19/18, and D. bardawil LB 2538) Cultivated under Laboratory Conditions. Journal of Bioscience and Bioengineering, 11(November). https://doi.org/10.1999/1307-6892/69056.
Baroli, I., & Melis, A. (1995). Photoinhibition and repair in Dunaliella salina acclimated to different growth irradiances. Planta, 198, 640–646.
Carvalho, A. P. A., Meireles, L. A. L. A., & Malcata, F. X. (2006). Microalgal reactors: a review of enclosed system designs and performances. Biotechnology Progress, 22(6), 1490–1506.
Ling, X., Weathers, P. J., Xiong, X.-R., & Liu, C.-Z. (2009). Microalgal bioreactors: challenges and opportunities. Engineering in Life Sciences, 9(3), 178–189.
Acién, F. G., Fernández, J. M., Magán, J. J., & Molina, E. (2012). Production cost of a real microalgae production plant and strategies to reduce it. Biotechnology Advances, 30(6), 1344–1353. https://doi.org/10.1016/j.biotechadv.2012.02.005.
Kroumov, A., Gacheva, G., Iliev, I., Alexandrov, S., Pilarski, P., & Petkov, G. (2013). Analysis of Sf/V ratio of photobioreactors linked with algal physiology. Genetics and. Plant Physiology, 3(1–2), 55–64.
Slegers, P. M. M., van Beveren, P. J. M. J. M., Wijffels, R. H. H., Van Straten, G., Van Boxtel, A. J. B., Van Straten, G., & Van Boxtel, A. J. B. (2013). Scenario analysis of large scale algae production in tubular photobioreactors. Applied Energy, 105, 395–406. https://doi.org/10.1016/j.apenergy.2012.12.068.
Fernández, I., Acién, F. G., Guzmán, J. L., Berenguel, M., & Mendoza, J. L. (2016). Dynamic model of an industrial raceway reactor for microalgae production. Algal Research, 17, 67–78. https://doi.org/10.1016/j.algal.2016.04.021.
Richmond, A. (2004). Principles for attaining maximal microalgal productivity in photobioreactors: an overview. Hydrobiologia, 512, 33–37. https://doi.org/10.1023/B:HYDR.0000020365.06145.36.
Briassoulis, D., Panagakis, P., Chionidis, M., Tzenos, D., Lalos, A., Tsinos, C., et al. (2010). An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresource Technology, 101(17). https://doi.org/10.1016/j.biortech.2010.03.103.
Torzillo, G., Pushparaj, B., Bocci, F., Balloni, W., Materassi, R., & Florenzano, G. (1986). Production of Spirulina biomass in closed photobioreactors. Biomass, 11, 61–74.
Behrens. (2005). Photobioreactors and fermentors: the light and dark sides of growing algae. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 189–204). San Diego: Elsevier.
Acién, F. G. G., Molina, E., Reis, A., Torzillo, G., Zittelli, G. C. C., Sepúlveda, C., & Masojídek, J. (2017). Photobioreactors for the production of microalgae. In Gonzalez-Fernandez, Cristina & Muñoz, R (Eds.), Microalgae-based biofuels and bioproducts: from feedstock cultivation to end-products (Vol. 12, pp. 1–44). Sawston: Woodhead Publishing. https://doi.org/10.1016/B978-0-08-101023-5.00001-7.
Díaz, J. P., Inostroza, C., & Acién Fernández, F. G. (2019). Fibonacci-type tubular photobioreactor for the production of microalgae. Process Biochemistry, 86, 1–8. https://doi.org/10.1016/j.procbio.2019.08.008.
Dixon, L. E., Hodge, S. K., Ooijen, G. Van, Troein, C., Akman, O. E., & Millar, A. J. (2014). Light and circadian regulation of clock components aids flexible responses to environmental signals.
Xu, Y., Ibrahim, I. M., & Harvey, P. J. (2016). The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiology and Biochemistry, 106, 305–315. https://doi.org/10.1016/j.plaphy.2016.05.021.
Pradeep Mohan Kumar, K., Vijayan, V., Suresh Kumar, B., Vivek, C. M., & Dinesh, S. (2018). Computational analysis and optimization of spiral plate heat exchanger. Journal of Applied Fluid Mechanics, 11(Specialissue), 121–128.
Ministerio de Energía. (2019). Explorador solar. Gobierno de Chile.
Jacobson, M. Z., & Jadhav, V. (2018). World estimates of PV optimal tilt angles and ratios of sunlight incident upon tilted and tracked PV panels relative to horizontal panels. Solar Energy, 169(April), 55–66. https://doi.org/10.1016/j.solener.2018.04.030.
Tredici, M. R., & Zlttelli, G. C. (1998). Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnology and Bioengineering, 57(2), 187–197. https://doi.org/10.1002/(SICI)1097-0290(19980120)57:2<187::AID-BIT7>3.0.CO;2-J.
Grima, E. M., Camacho, F. G., Pérez, J. A. S., Sevilla, J. M. F., Fernández, F. G. A., & Gómez, A. C. (1994). A mathematical model of microalgal growth in light-limited chemostat culture. Journal of Chemical Technology and Biotechnology, 61(2), 167–173. https://doi.org/10.1002/jctb.280610212.
Kroumov, A. D. (2013). Analysis of Sf/V ratio of photobioreactors linked with algal physiology. Genetics and. Plant Physiology, 3(1–2), 55–64.
Huang, Q., Jiang, F., Wang, L., & Yang, C. (2017). Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering, 3(3). https://doi.org/10.1016/J.ENG.2017.03.020.
Camacho-Rubio, F., Acién, F. G., Sánchez-Pérez, J. A., García-Camacho, F., & Molina-Grima, E. (1999). Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnology and Bioengineering, 62(1), 71–86. https://doi.org/10.1002/(SICI)1097-0290(19990105)62:1<71::AID-BIT9>3.0.CO;2-T.
Mendoza, J. L., Granados, M. R., de Godos, I., Acién, F. G., Molina, E., Heaven, S., & Banks, C. J. (2013). Oxygen transfer and evolution in microalgal culture in open raceways. Bioresource Technology, 137, 188–195.
Barceló-Villalobos, M., Guzmán Sánchez, J. L. L., Martín Cara, I., Sánchez Molina, J. A. A., & Acién Fernández, F. G. G. (2018). Analysis of mass transfer capacity in raceway reactors. Algal Research, 35(July), 91–97. https://doi.org/10.1016/j.algal.2018.08.017.
Camacho Rubio, F., Acien Fernandez, F. G., Sáchez Pérez, J. A., García Camacho, F., Molina Grima, E., & Alii, E. (1999). Prediction of dissolved oxygen and carbon dioxide concentrationprofiles in tubular photobioreactors for microalgal culture. Biotechnology and Bioengineering, 62(1) (FEBRUARY), 71–86. https://doi.org/10.1002/(SICI)1097-0290(19990105)62.
Scoma, A., Giannelli, L., Faraloni, C., & Torzillo, G. (2012). Outdoor H2 production in a 50-L tubular photobioreactor by means of a sulfur-deprived culture of the microalga Chlamydomonas reinhardtii. Journal of Biotechnology, 157(4), 620–627. https://doi.org/10.1016/j.jbiotec.2011.06.040.
Perner-Nochta, I., & Posten, C. (2007). Simulations of light intensity variation in photobioreactors. Journal of Biotechnology, 131(3), 276–285.
Oncel, S., & Sabankay, M. (2012). Microalgal biohydrogen production considering light energy and mixing time as the two key features for scale-up. Bioresource Technology, 121, 228–234. https://doi.org/10.1016/j.biortech.2012.06.079.
Richmond, A. (2013). Biological principles of mass cultivation of phototrophic microalgae. In R. Amos & Q. Hu (Eds.), Handbook of microalgal culture, applied phycology and biotechnology (pp. 171–204). Oxford: Wiley & Sons, Ltd.Blackwell Publishing Ltd.
Wang, B., Lan, C. Q., & Horsman, M. (2012). Closed photobioreactors for production of microalgal biomasses. Biotechnology Advances, 30(4), 904–912. https://doi.org/10.1016/j.biotechadv.2012.01.019.
Daliry, S., Hallajisani, A., Mohammadi Roshandeh, J., Nouri, H., & Golzary, A. (2017). Investigation of optimal condition for Chlorella vulgaris microalgae growth. Global Journal of Environmental Science and Management. https://doi.org/10.22034/gjesm.2017.03.02.010.
Cuaresma, M., Janssen, M., Vilchez, C., & Wiljffels, R. (2011). Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresource Technology, 102(8), 29–37.
Torzillo, G., & Zittelli, G. C. (2015). Tubular photobioreactors. In M. E. Zappi, A. Prokop, & R. K. Bajpai (Eds.), (pp. 187–212). Switzerland: Springer International Publishing.
Acién, F. G., Fernández, J. M., Molina Grima, E., & A, F. G. (2013). Photobioreactors for the production of microalgae. Reviews in Environmental Science and Biotechnology, 12(2), 131–151.
Masojídek, J., Malapascua, J. R., Kopecký, J., & Sergejevová, M. (2015). Thin-layer systems for mass cultivation of microalgae: flat panels and sloping cascades. In Algal biorefineries: volume 2: products and refinery design (pp. 237–261). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-20200-6_7.
Sierra, E., Acién, F. G., Fernández, J. M., García, J. L., González, C., & Molina, E. (2008). Characterization of a flat plate photobioreactor for the production of microalgae. Chemical Engineering Journal, 138(1–3), 136–147.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99(10), 4021–4028. https://doi.org/10.1016/j.biortech.2007.01.046.
Griffiths, M. J., & Harrison, S. T. L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21, 493–507. https://doi.org/10.1007/s10811-008-9392-7.
García-González, M., Moreno, J., Manzano, J. C., Florencio, F. J., Guerrero, M. G., García-González, M., et al. (2005). Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. Journal of Biotechnology, 115(1), 81–90. https://doi.org/10.1016/j.jbiotec.2004.07.010.
Weissman, J. C., & Products, R. P. G. M. (1987). Microalgal open pond systems for the purpose of producing fuels. Solar Energy Research Institute, 61, 1–231.
Costache, T. A., Acién Fernández, F. G., Morales, M. M., Fernández-Sevilla, J. M., Stamatin, I., & Molina, E. (2013). Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Applied Microbiology and Biotechnology, 97(17), 7627–7637. https://doi.org/10.1007/s00253-013-5035-2.
Ippoliti, D., González, A., Martín, I., Sevilla, J. M. F., Pistocchi, R., & Acién, F. G. (2016). Outdoor production of Tisochrysis lutea in pilot-scale tubular photobioreactors. Journal of Applied Phycology, 28(6), 3159–3166. https://doi.org/10.1007/s10811-016-0856-x.
Acknowledgements
We wish to thank the members of the FONDEF project, D04I1258: Marcela Avila, Elizabet Rojas and Pablo Barria. The authors are also very grateful to Attilio Gattavara and Masatoshi Futagawa for their generous cooperation and especially to Claudio Brieba (R.I.P.) for being part of this dream.
Funding
This research was supported by EU H2020 SABANA project from the European Union’s Horizon 2020 Research and Innovation program under the Grant Agreement No. 727874.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Díaz, J.P., Inostroza, C. & Acién, F.G. Scale-up of a Fibonacci-Type Photobioreactor for the Production of Dunaliella salina. Appl Biochem Biotechnol 193, 188–204 (2021). https://doi.org/10.1007/s12010-020-03410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03410-x