Abstract
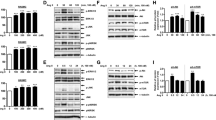
Farrerol, a dihydroflavone isolated from Rhododendron dauricum L., can inhibit vascular smooth muscle cell (VSMC) proliferation and exert a protective effect on H2O2-induced vascular endothelial cells injury. In this study, we investigated the effects of farrerol on VSMC phenotypic modulation and balloon injury-induced vascular neointimal formation and explored the underlying mechanisms. Serum-starved rat thoracic aorta SMCs (RASMCs) were first pretreated with farrerol (3, 10, and 30 μM, respectively), U0126 (a MEK kinase inhibitor), and SB203580 (a p38 kinase inhibitor), and followed by treatment with serum (10% FBS). The expression of several VSMC-specific markers, including α-SMA, SM22α, and OPN, were analyzed by western blot. Phosphorylation of extracellular signal-regulated protein kinase 1/2 (ERK 1/2) and p38 mitogen-activated protein kinase (MAPK) was also investigated. Farrerol inhibited the serum-induced transition of RASMCs from the contractile to the synthetic phenotype, and this was associated with a decrease in α-SMA and SM22α expression, and an increase in OPN expression. Farrerol also inhibited serum-induced phosphorylation of ERK1/2 and p38MAPK in RASMCs. Moreover, U0126 and SB203580 both inhibited the serum-induced phenotypic transition of RASMCs. These findings indicate that farrerol can maintain the contractile phenotype of VSMCs partly via inactivating the ERK1/2 and p38 MAPK signaling pathways. Using a rat model of carotid artery balloon injury, inhibition of VSMC phenotypic transition and suppression of neointimal formation were confirmed in vivo following the perivascular application of farrerol. Our results suggested that farrerol could be a promising lead compound for the treatment of vascular proliferative diseases.







Similar content being viewed by others
Data availability
All data sets supporting the results of this article are included within this published article.
References
Bauters C, Isner JM (1997) The biology of restenosis. Prog Cardiovasc Dis 40(2):107–116. https://doi.org/10.1016/S0033-0620(97)80003-5
Kester M, Waybill P, Kozak M (2001) New strategies to prevent restenosis. Am J Cardiovasc Drugs 1(2):77–83. https://doi.org/10.2165/00129784-200101020-00001
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637. https://doi.org/10.1038/nm.3866
Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, Fong R, Woo YJ, Liu B, Montgomery SB, Wu JC, Zhu K, Chang R, Alamprese M, Tallquist MD, Kim JB, Quertermous T (2019) Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med 25(8):1280–1289. https://doi.org/10.1038/s41591-019-0512-5
Araim O, Ballantyne J, Waterhouse AL, Sumpio BE (2002) Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J Vas Surg 35(6):1226–1232. https://doi.org/10.1067/mva.2002.124358
Du JR, Li X, Zhang R, Qian ZM (2005) Tanshinone inhibits intimal hyperplasia in the ligated carotid artery in mice. J Ethnopharmacol 98(3):319–322. https://doi.org/10.1016/j.jep.2005.01.038
Guan S, Tang Q, Liu W, Zhu R, Li B (2014) Nobiletin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in a rat carotid artery injury model. Drug Dev Res 75(8):489–496. https://doi.org/10.1002/ddr.21230
Li F, Shang XK, Du XL, Chen S (2018) Rapamycin treatment attenuates angiotensin II -induced abdominal aortic aneurysm formation via VSMC phenotypic modulation and down-regulation of ERK1/2 activity. Curr Med Sci 38(1):93–100. https://doi.org/10.1007/s11596-018-1851-z
Li Q, Chen L, Zhu Y, Zhang M, Wang Y, Wang M (2011) Involvement of estrogen receptor-β in farrerol inhibition of rat thoracic aorta vascular smooth muscle cell proliferation. Acta Pharmacol Sin 32(4):433–440. https://doi.org/10.1038/aps.2011.1
Lai Y, Zeng H, He M, Qian H, Wu Z, Luo Z, Xue Y, Yao G, Zhang Y (2016) 6,8-Di-C-methyl-flavonoids with neuroprotective activities from Rhododendron fortunei. Fitoterapia 112:237–243. https://doi.org/10.1016/j.fitote.2016.06.008
Liu EL, Li J, Shi S, Wang X, Liang T, Wu B, Li Q (2016) Sustained ERK activation-mediated proliferation inhibition of farrerol on human gastric carcinoma cell line by G0/G1-phase cell-cycle arrest. Eur J Cancer Prev 2(6):490–499. https://doi.org/10.1097/CEJ.0000000000000212
Qin X, Hou X, Zhang M, Liang T, Zhi J, Han L, Li Q (2014) Relaxation of rat aorta by farrerol correlates with potency to reduce intracellular calcium of VSMCs. Int J Mol Sci 15(4):6641–6656. https://doi.org/10.3390/ijms15046641
Qin X, Hou X, Liang T, Chen L, Lu T, Li Q (2015) Farrerol can attenuate the aortic lesion in spontaneously hypertensive rats via the upregulation of eNOS and reduction of NAD(P)H oxidase activity. Eur J Pharmacol 769:211–218. https://doi.org/10.1016/j.ejphar.2015.11.020
Li J, Ge R, Tang L, Li Q (2013) Protective effects of farrerol against hydrogen-peroxide-induced apoptosis in human endothelium-derived EA.hy926 cells. Can J Physiol Pharmacol 91(9):733–740. https://doi.org/10.1139/cjpp-2013-0008
Li J, Ge R, Zhao C, Tang L, Li J, Li Q (2014) Farrerol regulates occludin expression in hydrogen peroxide-induced EA.hy926 cells by modulating ERK1/2 activity. Eur J Pharmacol 734:9–14. https://doi.org/10.1016/j.ejphar.2014.03.054
Liu E, Liang T, Wang X, Ban S, Han L, Li Q (2015) Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. Eur J Cancer Prev 24(5):365–372. https://doi.org/10.1097/CEJ.0000000000000104
Han M, Wen JK, Zheng B, Cheng Y, Zhang C (2006) Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am J Physiol Cell Physiol 291(1):50–58. https://doi.org/10.1152/ajpcell.00524.2005
Shafiq N, Malhotra S, Pandhi P, Grover A, Uboweja A (2005) A meta-analysis of clinical trials of paclitaxel- and sirolimus-eluting stents in patients with obstructive coronary artery disease. Br J Clin Pharmacol 59(1):94–101. https://doi.org/10.1111/j.1365-2125.2005.02258.x
Shao PL, Chiu CC, Yuen CM, Chua S, Chang LT, Sheu JJ, Sun CK, Wu CJ, Wang CJ, Yip HK (2010) Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology 115(2):130–144. https://doi.org/10.1159/000262331
Kirchmer MN, Franco A, Albasanz-Puig A, Murray J, Yagi M, Gao L, Dong ZM, Wijelath ES (2014) Modulation of vascular smooth muscle cell phenotype by STAT-1 and STAT-3. Atherosclerosis 234(1):169–175. https://doi.org/10.1016/j.atherosclerosis.2014.02.029
Zhang YN, Xie BD, Sun L, Chen W, Jiang SL, Liu W, Bian F, Tian H, Li RK (2016) Phenotypic switching of vascular smooth muscle cells in the 'normal region' of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med 20(6):1049–1061. https://doi.org/10.1111/jcmm.12825
Karaarslan K, Abud B, Albayrak G, Aykut K, Ergür BU, Silistreli E (2015) The effect of resveratrol on intimal hyperplasia and endothelial proliferation of rabbit carotid artery anastomosis. Interact Cardiovasc Thorac Surg 20(1):15–20. https://doi.org/10.1093/icvts/ivu316
Han M, Dong LH, Zheng B, Shi JH, Wen JK, Cheng Y (2009) Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci 84(13–14):394–401. https://doi.org/10.1016/j.lfs.2008.11.017
Ci XX, Lv HM, Wang LD, Wang XW, Peng LP, Qin XF, Cheng GH (2015) The antioxidative potential of farrerol occurs via the activation of Nrf2 mediated HO-1 signaling in RAW 2647 cells. Chem Biol Interactions 239(2015):192–199. https://doi.org/10.1016/j.cbi.2015.06.032
Shi L, Feng XE, Cui JR, Fang LH, Du GH, Li QS (2010) Synthesis and biological activity of flavanone derivatives. Bioorg Med Chem Lett 20(18):5466–5468. https://doi.org/10.1016/j.bmcl.2010.07.090
Ma X, Wang Y, Stephens NL (1998) Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am J Physiol 274(5):C1206–C1214. https://doi.org/10.1152/ajpcell.1998.274.5.C1206
Yu QH, Li W, Jin R, Yu SY, Xie DW, Zheng XC, Zhong W, Cheng X, Hu SB, Li M, Zheng QC, Li GH, Song ZF (2019) PI3Kγ regulates vascular smooth muscle cell phenotypic modulation and neointimal formation through CREB/YAP signaling. Arterioscler Thromb Vasc Biol 39(3):e91–e105. https://doi.org/10.1161/ATVBAHA.118.312212
Zhang L, Xu Z, Wu Y, Liao J, Zeng F, Shi L (2018) Akt/eNOS and MAPK signaling pathways mediated the phenotypic switching of thoracic aorta vascular smooth muscle cells in aging/hypertensive rats. Physiol Res 67(4):543–553. https://doi.org/10.33549/physiolres.933779
Longchamp A, Allagnat F, Alonso F, Kuppler C, Dubuis C, Ozaki CK, Mitchell JR, Berceli S, Corpataux JM, Déglise S, Haefliger JA (2015) Connexin43 inhibition prevents human vein grafts intimal hyperplasia. PLoS ONE 10(9):e0138847. https://doi.org/10.1371/journal.pone.0138847
Cheung AK, Terry C, Li L (2008) Pathogensis and local drug delivery for prevention of vascular access stenosis. J Ren Nutr 18(1):140–145. https://doi.org/10.1053/j.jrn.2007.10.028
Myllärniemi M, Frösen J, Calderón Ramirez LG, Buchdunger E, Lemström K, Häyry P (1999) Selective tyrosine kinase inhibitor for the platelet-derived growth factor receptor in vitro inhibits smooth muscle cell proliferation after reinjury of arterial intima in vivo. Cardiovasc Drugs Ther 13(2):159–168. https://doi.org/10.1023/a:1007700629728
Fujinaga K, Onoda K, Yamamoto K, Imanaka-Yoshida K, Takao M, Shimono T, Shimpo H, Yoshida T, Yada I (2004) Locally applied cilostazol suppresses neointimal hyperplasia by inhibiting tenascin-c synthesis and smooth muscle cell proliferation in free artery grafts. J Thorac Cardiovasc Surg 128(3):357–363. https://doi.org/10.1016/j.jtcvs.2003.11.015
Schachner T, Zou Y, Oberhuber A, Tzankov A, Mairinger T, Laufer G, Bonatti JO (2004) Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg 77(5):1580–1585. https://doi.org/10.1016/j.athoracsur.2003.10.008
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81172938), the Natural Science Foundation for Young Scientists of Shanxi Province (201601D021158); and the Shanxi Key Laboratory of Innovative Drug for the Treatment of Serious Diseases Basing on the Chronic Inflammation Shanxi University of Chinese Medicine (SXIDL-2018–03).
Author information
Authors and Affiliations
Contributions
QL, EL, and TL conceived and designed the experiments. EL, SS, and JL performed the experiments. EL wrote the manuscript. EL and RG performed the data analysis. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical approval
All the authors have read and approved to submit this manuscript to Molecular and Cellular Biochemistry.
Consent for publication
All the authors warrant that this article is the authors' original work, has not received prior publication, and is not under consideration for publication else where.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, E., Shi, S., Li, J. et al. Farrerol maintains the contractile phenotype of VSMCs via inactivating the extracellular signal-regulated protein kinase 1/2 and p38 mitogen-activated protein kinase signaling. Mol Cell Biochem 475, 249–260 (2020). https://doi.org/10.1007/s11010-020-03878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03878-5