Abstract
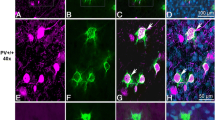
The 22q11.2 deletion has been identified as a risk factor for multiple neurodevelopmental disorders. Behavioral and cognitive impairments are common among carriers of the 22q11.2 deletion. Parvalbumin expressing (PV+) interneurons provide perisomatic inhibition of excitatory neuronal circuits through GABAA receptors, and a deficit of PV+ inhibitory circuits may underlie a multitude of the behavioral and functional deficits in the 22q11.2 deletion syndrome. We investigated putative deficits of PV+ inhibitory circuits and the associated molecular, morphological, and functional alterations in the prefrontal cortex (PFC) of the Df(h22q11)/+ mouse model of the 22q11.2 hemizygous deletion. We detected a significant decrease in the number of PV+ interneurons in layers II/III of PFC in Df(h22q11)/+ mice together with a reduction in the mRNA and protein levels of GABAA (α3), a PV+ putative postsynaptic receptor subunit. Pyramidal neurons from the same layers further experienced morphological reorganizations of spines and dendrites. Accordingly, a decrease in the levels of the postsynaptic density protein 95 (PSD95) and a higher neuronal activity in response to the GABAA antagonist bicuculline were measured in these layers in PFC of Df(h22q11)/+ mice compared with their wild-type littermates. Our study shows that a hemizygotic deletion of the 22q11.2 locus leads to deficit in the GABAergic control of network activity and involves molecular and morphological changes in both the inhibitory and excitatory synapses of parvalbumin interneurons and pyramidal neurons specifically in layers II/III PFC.



Similar content being viewed by others
Data Availability
Data are available upon request.
References
Meechan DW, Maynard TM, Gopalakrishna D, Wu Y, LaMantia AS (2007) When half is not enough: gene expression and dosage in the 22q11 deletion syndrome. Gene Expr 13(6):299–310
Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, Antaki D, Shetty A et al (2017) Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 49(1):27–35. https://doi.org/10.1038/ng.3725
Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C (2009) Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res Dev Disabil 30(4):763–773. https://doi.org/10.1016/j.ridd.2008.10.007
Chow EW, Watson M, Young DA, Bassett AS (2006) Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res 87(1–3):270–278. https://doi.org/10.1016/j.schres.2006.04.007
Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E (2001) Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med 3(1):34–39. https://doi.org/10.1097/00125817-200101000-00008
Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P et al (2008) Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet 40(6):751–760. https://doi.org/10.1038/ng.138
Paylor R, McIlwain KL, McAninch R, Nellis A, Yuva-Paylor LA, Baldini A, Lindsay EA (2001) Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet 10(23):2645–2650. https://doi.org/10.1093/hmg/10.23.2645
Sakurai T, Gamo NJ, Hikida T, Kim SH, Murai T, Tomoda T, Sawa A (2015) Converging models of schizophrenia--network alterations of prefrontal cortex underlying cognitive impairments. Prog Neurobiol 134:178–201. https://doi.org/10.1016/j.pneurobio.2015.09.010
Wood JN, Romero SG, Makale M, Grafman J (2003) Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci 15(2):236–248. https://doi.org/10.1162/089892903321208178
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE (2012) GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62(3):1574–1583. https://doi.org/10.1016/j.neuropharm.2011.01.022
Yu Z, Fang Q, Xiao X, Wang YZ, Cai YQ, Cao H, Hu G, Chen Z et al (2013) GABA transporter-1 deficiency confers schizophrenia-like behavioral phenotypes. PLoS One 8(7):e69883. https://doi.org/10.1371/journal.pone.0069883
Freund TF, Katona I (2007) Perisomatic inhibition. Neuron 56(1):33–42. https://doi.org/10.1016/j.neuron.2007.09.012
Lagler M, Ozdemir AT, Lagoun S, Malagon-Vina H, Borhegyi Z, Hauer R, Jelem A, Klausberger T (2016) Divisions of identified parvalbumin-expressing basket cells during working memory-guided decision making. Neuron 91(6):1390–1401. https://doi.org/10.1016/j.neuron.2016.08.010
Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247):663–667. https://doi.org/10.1038/nature08002
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459(7247):698–702. https://doi.org/10.1038/nature07991
Ferguson BR, Gao WJ (2018) PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits 12:37. https://doi.org/10.3389/fncir.2018.00037
Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KL, Foggetti A, Crouch B, Riedel G, Wulff P (2015) Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep 5:16778. https://doi.org/10.1038/srep16778
da Silva AF, Boot E, Schmitz N, Nederveen A, Vorstman J, Lavini C, Pouwels PJ, de Haan L et al (2011) Proton magnetic resonance spectroscopy in 22q11 deletion syndrome. PLoS One 6(6):e21685. https://doi.org/10.1371/journal.pone.0021685
Philip N, Bassett A (2011) Cognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndrome. Behav Genet 41(3):403–412. https://doi.org/10.1007/s10519-011-9468-z
Didriksen M, Fejgin K, Nilsson SR, Birknow MR, Grayton HM, Larsen PH, Lauridsen JB, Nielsen V et al (2017) Persistent gating deficit and increased sensitivity to NMDA receptor antagonism after puberty in a new mouse model of the human 22q11.2 microdeletion syndrome: a study in male mice. J Psychiatry Neurosci 42(1):48–58. https://doi.org/10.1503/jpn.150381
Nilsson SRO, Heath CJ, Takillah S, Didienne S, Fejgin K, Nielsen V, Nielsen J, Saksida LM et al (2018) Continuous performance test impairment in a 22q11.2 microdeletion mouse model: Improvement by amphetamine. Transl Psychiatry 8:247. https://doi.org/10.1038/s41398-018-0295-3
Tripathi A, Spedding M, Schenker E, Didriksen M, Cressant A, Jay TM (2020) Cognition- and circuit-based dysfunction in a mouse model of 22q11.2 microdeletion syndrome: effects of stress. Transl Psychiatry 10(1):41. https://doi.org/10.1038/s41398-020-0687-z
Gundersen HJ (2002) The smooth fractionator. J Microsc 207(Pt 3):191–210. https://doi.org/10.1046/j.1365-2818.2002.01054.x
Van De Werd HJ, Uylings HB (2014) Comparison of (stereotactic) parcellations in mouse prefrontal cortex. Brain Struct Funct 219(2):433–459. https://doi.org/10.1007/s00429-013-0630-7
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64(15):5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496
Van De Werd HJJM, Rajkowska G, Evers P, Uylings HBM (2010) Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct 214(4):339–353. https://doi.org/10.1007/s00429-010-0247-z
Al-Absi AR, Christensen HS, Sanchez C, Nyengaard JR (2018) Evaluation of semi-automatic 3D reconstruction for studying geometry of dendritic spines. J Chem Neuroanat 94:119–124. https://doi.org/10.1016/j.jchemneu.2018.10.008
Paxinos G, Franklin KBJ, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Yger P, Spampinato GL, Esposito E, Lefebvre B, Deny S, Gardella C, Stimberg M, Jetter F et al (2018) A spike sorting toolbox for up to thousands of electrodes validated with ground truth recordings in vitro and in vivo. Elife 7. https://doi.org/10.7554/eLife.34518
Beneyto M, Abbott A, Hashimoto T, Lewis DA (2011) Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex 21(5):999–1011. https://doi.org/10.1093/cercor/bhq169
Berggaard N, Seifi M, van der Want JJL, Swinny JD (2018) Spatiotemporal distribution of GABAA receptor subunits within layer II of mouse medial entorhinal cortex: implications for grid cell excitability. Front Neuroanat 12:46. https://doi.org/10.3389/fnana.2018.00046
Lewis DA, Curley AA, Glausier JR, Volk DW (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35(1):57–67. https://doi.org/10.1016/j.tins.2011.10.004
Chattopadhyaya B, Cristo GD (2012) GABAergic circuit dysfunctions in neurodevelopmental disorders. Front Psychiatry 3:51. https://doi.org/10.3389/fpsyt.2012.00051
Lawrence YA, Kemper TL, Bauman ML, Blatt GJ (2010) Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand 121(2):99–108. https://doi.org/10.1111/j.1600-0404.2009.01234.x
Buzsaki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ (2007) GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54(6):889–903. https://doi.org/10.1016/j.neuron.2007.05.015
Larsen KM, Pellegrino G, Birknow MR, Kjaer TN, Baare WFC, Didriksen M, Olsen L, Werge T et al (2018) 22q11.2 deletion syndrome is associated with impaired auditory steady-state gamma response. Schizophr Bull 44(2):388–397. https://doi.org/10.1093/schbul/sbx058
Lewis DA, Cruz DA, Melchitzky DS, Pierri JN (2001) Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry 158(9):1411–1422. https://doi.org/10.1176/appi.ajp.158.9.1411
Hoftman GD, Datta D, Lewis DA (2017) Layer 3 excitatory and inhibitory circuitry in the prefrontal cortex: developmental trajectories and alterations in schizophrenia. Biol Psychiatry 81(10):862–873. https://doi.org/10.1016/j.biopsych.2016.05.022
Nathanson AJ, Davies PA, Moss SJ (2019) Inhibitory synapse formation at the axon initial segment. Front Mol Neurosci 12:266. https://doi.org/10.3389/fnmol.2019.0026641
Lewis DA (2012) Cortical circuit dysfunction and cognitive deficits in schizophrenia—implications for preemptive interventions. Eur J Neurosci 35(12):1871–1878. https://doi.org/10.1111/j.1460-9568.2012.08156.x
Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM (2006) Altered expression of alpha3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain 129(Pt 12):3277–3289. https://doi.org/10.1093/brain/awl287
Niturad CE, Lev D, Kalscheuer VM, Charzewska A, Schubert J, Lerman-Sagie T, Kroes HY, Oegema R et al (2017) Rare GABRA3 variants are associated with epileptic seizures, encephalopathy and dysmorphic features. Brain 140(11):2879–2894. https://doi.org/10.1093/brain/awx236
Fiorelli R, Rudolph U, Straub CJ, Feldon J, Yee BK (2008) Affective and cognitive effects of global deletion of alpha 3-containing gamma-aminobutyric acid-A receptors. Behav Pharmacol 19(5–6):582–596. https://doi.org/10.1097/FBP.0b013e32830dc0c7
Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D (2008) Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry 165(12):1585–1593. https://doi.org/10.1176/appi.ajp.2008.08030395
Mukherjee A, Carvalho F, Eliez S, Caroni P (2019) Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell 178(6):1387–1402 e1314. https://doi.org/10.1016/j.cell.2019.07.023
Kimoto S, Muraki K, Toritsuka M, Mugikura S, Kajiwara K, Kishimoto T, Illingworth E, Tanigaki K (2012) Selective overexpression of Comt in prefrontal cortex rescues schizophrenia-like phenotypes in a mouse model of 22q11 deletion syndrome. Transl Psychiatry 2:e146. https://doi.org/10.1038/tp.2012.70
Mohler H (2007) Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem 102(1):1–12. https://doi.org/10.1111/j.1471-4159.2007.04454.x
Melchitzky DS, Sesack SR, Pucak ML, Lewis DA (1998) Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol 390(2):211–224. https://doi.org/10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4
Sudhof TC, Malenka RC (2008) Understanding synapses: past, present, and future. Neuron 60(3):469–476. https://doi.org/10.1016/j.neuron.2008.10.011
Glausier JR, Lewis DA (2013) Dendritic spine pathology in schizophrenia. Neuroscience 251:90–107. https://doi.org/10.1016/j.neuroscience.2012.04.044
Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57(1):65–73. https://doi.org/10.1001/archpsyc.57.1.65
Coley AA, Gao WJ (2018) PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuro-Psychopharmacol Biol Psychiatry 82:187–194. https://doi.org/10.1016/j.pnpbp.2017.11.016
Mukai J, Tamura M, Fenelon K, Rosen AM, Spellman TJ, Kang R, MacDermott AB, Karayiorgou M et al (2015) Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 86(3):680–695. https://doi.org/10.1016/j.neuron.2015.04.003
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 345(6196):1255263. https://doi.org/10.1126/science.1255263
Marowsky A, Rudolph U, Fritschy JM, Arand M (2012) Tonic inhibition in principal cells of the amygdala: a central role for alpha 3 subunit-containing GABA(A) receptors. J Neurosci 32(25):8611–8619. https://doi.org/10.1523/Jneurosci.4404-11.2012
Acknowledgments
We thank Helene M. Andersen and Jan Jacobsen for excellent technical assistance and Prof. Dr. Ulrich Egert (Bernstein Center Freiburg, Freiburg University) and Szilard Sajgo (Department of Biomedicine, Aarhus University) for their valuable assistance with the acquisition and the analysis of data from the HD-MEA.
Contributions
A.R.A. performed the experiments, A.R.A., P.Q., S.O., and A.R.K. analyzed and interpreted the results. The first draft of the manuscript was written by A.R.A. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Department of Clinical Medicine at Aarhus University, Henny Sophie Clausen og møbelarkitekt Aksel Clausens Fond, and Centre for Stochastic Geometry, and the latter is supported by Villum Foundation (no specified grant number). The funders had no role in design of this study, analysis and interpretation of data, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All studies were carried out based on the approval from the Danish Animal Experiments Inspectorate (2012-15-2934-00254).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 956 kb)
Rights and permissions
About this article
Cite this article
Al-Absi, AR., Qvist, P., Okujeni, S. et al. Layers II/III of Prefrontal Cortex in Df(h22q11)/+ Mouse Model of the 22q11.2 Deletion Display Loss of Parvalbumin Interneurons and Modulation of Neuronal Morphology and Excitability. Mol Neurobiol 57, 4978–4988 (2020). https://doi.org/10.1007/s12035-020-02067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02067-1