Abstract
Co-pyrolysis of biomass biopolymers (lignin and cellulose) with plastic wastes (polyethylene and polystyrene) coupled with downstream catalytic steam reforming of the pyrolysis gases for the production of a hydrogen-rich syngas is reported. The catalyst used was 10 wt.% nickel supported on MCM-41. The influence of the process parameters of temperature and the steam flow rate was examined to optimize hydrogen and syngas production. The cellulose/plastic mixtures produced higher hydrogen yields compared with the lignin/plastic mixtures. However, the impact of raising the catalytic steam reforming temperature from 750 to 850 °C was more marked for lignin addition. For example, the hydrogen yield for cellulose/polyethylene at a catalyst temperature of 750 °C was 50.3 mmol g−1 and increased to 60.0 mmol g−1 at a catalyst temperature of 850 °C. However, for the lignin/polyethylene mixture, the hydrogen yield increased from 25.0 to 50.0 mmol g−1 representing a twofold increase in hydrogen yield. The greater influence on hydrogen and yield for the lignin/plastic mixtures compared to the cellulose/plastic mixtures is suggested to be due to the overlapping thermal degradation profiles of lignin and the polyethylene and polystyrene. The input of steam to the catalyst reactor produced catalytic steam reforming conditions and a marked increase in hydrogen yield. The influence of increased steam input to the process was greater for the lignin/plastic mixtures compared to the cellulose/plastic mixtures, again linked to the overlapping thermal degradation profiles of the lignin and the plastics. A comparison of the Ni/MCM-41 catalyst with Ni/Al2O3 and Ni/Y-zeolite-supported catalysts showed that the Ni/Al2O3 catalyst gave higher yields of hydrogen and syngas.
Graphic abstract

Similar content being viewed by others
Introduction
Hydrogen is an important commodity that can be used to generate clean energy in fuel cells and hydrogen engines and is also used in the petroleum and chemical industries, and iron and steel plants. About 95% of hydrogen is currently produced from fossil fuels, mainly from natural gas by the catalytic steam reforming process [1]. The production of hydrogen from renewable resources such as biomass offers a sustainable and renewable source of energy with abundant readily available feedstocks including agricultural residues, forestry residues, and municipal solid waste [2,3,4,5,6]. Waste plastics are also a readily available resource that has been used for the production of hydrogen [7,8,9]. The hydrogen yield from biomass is relatively low compared to waste plastics because of the lower hydrogen content and the presence of high concentrations of oxygen in the biomass; the higher H:C ratio of plastics off-setting the high oxygen content of biomass [10]. Therefore, co-processing waste plastics with biomass to enhance the overall production of hydrogen has been investigated by several researchers [11,12,13,14,15]. The advantage of co-processing waste plastics with biomass is reported to produce a higher total yield of gas, increased yield of hydrogen, and reduced catalyst deactivation from carbonaceous coke deposits compared to biomass processing alone [15,16,17].
The commercial production of hydrogen from natural gas involves catalytic steam reforming of the methane using nickel-based catalysts to produce hydrogen and carbon monoxide [5]. The commercial process has been used as the basis for the production of hydrogen from both biomass and waste plastics, whereby the methane is substituted by hydrocarbon gases produced from the pyrolysis of biomass and/or waste plastics. Several researcher groups have used a two-stage process of pyrolysis followed by catalytic steam reforming in two separate temperature-controlled reactors for biomass [5, 18] and waste plastics [8, 19,20,21]. The two-stage process has several advantages, including improved interaction between pyrolysis gases and the catalyst, separate control of the pyrolysis and catalytic process conditions, and the reacted catalysts can be easily recovered for subsequent regeneration and reuse [7].
The most common catalysts used for hydrogen production from biomass [22] and waste plastics [23] are nickel-based, reflecting the main type of catalyst used for commercial methane catalytic steam reforming [24]. Other transition metals investigated include Co, Fe, Cu, and also noble metal catalysts such as Pt, Pd, Rh, and Ru [9, 24]. However, Ni-based catalysts, are preferred due to their lower cost and enhanced activity for breaking C─C, C─H, C─O, and O─H bonds and also for hydrogenation producing H atoms to form H2 [9]. The disadvantage of nickel as the active metal lies in its deactivation due to carbonaceous coke deposits on the surface through hydrocarbon decomposition and the Boudouard reaction leading to the covering of the active metal [25, 26]. Molybdenum carbide catalysts are active for catalytic steam reforming of hydrocarbons (methane) but with very low or zero-carbon formation on the catalyst [26, 27]. Liu et al. [28] used a Ni–Mo-sepiolite catalyst and reported reduced catalyst carbon formation for the catalytic steam reforming of a bio-oil aqueous fraction. Figen and Baykara [29] have reported that molybdenum-based catalysts exhibit activity for syngas production that is comparable to activities of noble metal catalysts. Therefore, it is interesting to compare the Ni/MCM-41 catalyst with molybdenum as a transition metal-based catalyst for the pyrolysis–catalytic steam reforming of the biopolymer/plastic mixtures. Besides, the support for the nickel-based catalysts has commonly been alumina (Al2O3) due to its mechanical strength and chemical resistance. However, in attempts to improve the stability of Ni-based catalysts, different support materials including highly porous zeolites and mesoporous MCM-41 have been investigated for hydrogen production from waste plastics [21, 30] and biomass [31]. It has been reported that the pore structure design allows for active metals to be located within the pore structure or nano-metal particles prevent pore-blocking during catalyst coking [9]. Therefore, using MCM-41 as the metal-catalyst support material offers a promising route to higher hydrogen production and is worthy of further investigation. However, using Ni-MCM-41 catalysts for hydrogen production from waste plastics should be compared with the more common catalyst supports, particularly Ni/Al2O3 or Ni/zeolite catalyst support materials.
Biomass is composed of three major biopolymers: cellulose (typically 40–50 wt.%), hemicellulose (15–30 wt.%), and lignin (15–30 wt.%) [32]. The thermal decomposition process of the biopolymers is an important factor that determines the proportion of each product fraction (gases, liquid and char) and their composition. Cellulose is a long-chain polysaccharide formed by D-glucose units, linked by β-1,4 glycosidic bonds. Hemicellulose is a complex, branched, and heterogeneous polymeric network, based on pentoses such as xylose and arabinose, hexoses such as glucose, mannose and galactose, and sugar acids. Lignin is a complex heteropolymer consisting of hydroxycinnamyl alcohols [33]. We have previously investigated the pyrolysis–catalytic steam reforming of cellulose, hemicellulose, and lignin, and reported that lignin produced the most hydrogen compared to cellulose and hemicellulose at 25.25 mmol g−1 [5]. The chemical structure of cellulose and hemicellulose are somewhat similar but very different from lignin. Therefore, to determine any major difference between the co-processing of biopolymer components of biomass and waste plastics for hydrogen production, it may be suggested that a comparison of cellulose with lignin would show this well.
In this paper, the biopolymer cellulose or lignin was co-pyrolysed with polyethylene and/or polystyrene in a two-stage, pyrolysis–catalytic steam reforming process to produce hydrogen. The catalyst used was 10 wt.% Ni/MCM-41 and the influence of catalyst temperature and the amount of input steam were investigated about the yield of hydrogen and also syngas (H2 and CO). Finally, the Ni/MCM-41 catalyst was compared with nickel catalysts supported on Al2O3 and Y-zeolite for comparison of hydrogen yield.
Materials and methods
Materials
Cellulose and lignin (Kraft) were obtained from Sigma-Aldrich, UK Ltd. Polyethylene (high-density polyethylene (HDPE) and polystyrene (PS) were obtained from Regain Polymers Ltd., UK. For the experiments, the biomass and plastics were crushed and sieved to give an average particle size of ~ 200 µm and intimately mixed for experimentation. Elemental analysis of the feedstocks was carried out using a Thermo EA2000 analyser. Proximate analysis was determined using a Shimadzu TGA-50 thermogravimetric analyser. The results for elemental and proximate analyses of the cellulose and lignin are shown in Table 1. The thermal decomposition profiles of the individual feedstock materials were also characterised using the Shimadzu TGA-50 thermogravimetric analyser.
The 10 wt.% of Ni/MCM-41 catalyst used for conducting the catalytic steam reforming experiments was prepared by an incipient wetness method. An aqueous solution of nickel hexa-nitrate was dissolved in 20 mL deionised water and stirred continuously for 30 min and then heated slowly to 90 °C with the MCM-41 support until a semi-solid slurry mixture was formed. The precursor slurry was dried in an oven overnight at 105 °C until all of the excess water was evaporated. The prepared catalyst was then calcined under an air atmosphere at a temperature regime of ambient temperature to 750 °C with a heating rate of 20 °C min−1 for and held at 750 °C for 3 h. The catalyst was ground and sieved to a particle size of 50–212 µm. The prepared catalyst was reduced under 95% of nitrogen with a 5% balance of hydrogen in a reduction furnace held at 800 °C.
Pyrolysis–catalytic steam reforming reactor system
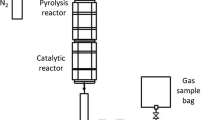
The reactor used for pyrolysis–catalytic steam reforming of the biopolymers and plastic mixtures was a two-stage, separately heated stainless steel reactor system, shown as a schematic diagram in Fig. 1. Details of the reactor system and experimental procedure have been detailed previously [5]. The reactor was 250 mm × 30 mm externally electrically heated with the pyrolysis of the feedstock (2 g) in the first stage followed by catalytic steam reforming (1.0 g of catalyst) of the evolved pyrolysis gases in the second stage. Water injection into the second stage produced catalytic steam reforming. The feedstock mixture of biopolymers and plastics was heated at 20 °C min−1 to the final pyrolysis temperature of 550 °C and held at that temperature for 30 min. The Ni/MCM-41 catalyst bed temperature was maintained at the desired temperature (either 750 °C or 850 °C). Nitrogen was used as carrier gas. Condensable liquids were collected in an air-cooled and solid dry ice (CO2)-cooled condenser system and non-condensable gases were collected in a Tedlar gas sample bag. All experiments were repeated for accuracy with negligible differences between the repeated experiments. Results were the average of the repeated experiments.
The collected gases were analysed using three Varian Ltd (UK) CP 3330 Gas Chromatography (GC) packed column gas chromatography. One GC analysed CO, H2, N2, and O2, with a 60–80 mesh molecular sieve size column, CO2 was analysed with a different GC with a 60–80 mesh molecular sieve, but with different chromatographic conditions to isolate the CO2. The third GC analysed C1─C4 hydrocarbons with an 80–100 mesh HayeSep column with a flame ionisation detector. Further details of the gas analysis methodology have been reported before [5].
Results and discussion
Thermogravimetric analysis (TGA) of feedstocks
The thermogravimetric analysis (TGA) analysis of the individual feedstock as well as the mixtures of biopolymers (cellulose, lignin) and plastics (HDPE and PS) was conducted to identify the mass loss profiles of the feedstocks under pyrolysis conditions. Figure 2 shows the TGA and the differential weight loss (DTG) of the biopolymers and plastics. Figure 2a, b shows that the cellulose, polystyrene, and polyethylene have peak decomposition temperatures of 350 °C, 430 °C, and 490 °C, respectively, allowing clear differentiation of these feedstocks in terms of their thermal degradation profiles. However, lignin degrades over a wider temperature range of about 200 °C to 750 °C. Similar thermal decomposition profiles have been reported by others [17, 34, 35]. Figure 2c, d shows the thermal degradation profiles of the mixtures of cellulose and lignin with the polyethylene and polystyrene plastics. The biopolymers and plastics were mixed in a 1:1 ratio. The TGA and DTG thermal degradation profiles reflect the individual components in the mixtures; however, there was evidence of some interaction of the components suggested by a small shift to lower decomposition temperatures of about 5 °C for the decomposition of cellulose with polyethylene and polystyrene. Oyedun et al. [36] have also reported that for the thermogravimetric decomposition of mixtures of biomass with plastics (polystyrene and polyethylene), there is a shift to a lower temperature for the onset of thermal degradation of the plastic. They also suggested that polystyrene had a greater interaction with the biomass compared to polyethylene which was closely linked to the lignin decomposition. In addition, the overlapping thermal degradation profile of lignin and polystyrene enhances interaction. Sharypov et al. [37] investigated the co-pyrolysis of wood biomass and plastic mixtures via thermogravimetric analysis and reported no significant interaction between the biomass and plastics. But, they also investigated laboratory scale pyrolysis of the mixtures and reported that interaction between biomass and plastics produced a higher yield of lower molecular weight hydrocarbons than would be expected based on the pyrolysis of the individual feedstock materials. Similarly, Burra and Gupta [38] used thermogravimetric analysis of mixtures of biomass with different plastics and found no interaction between biomass and polypropylene. However, for larger-scale experimental work in a steam gasification reactor, the interaction between biomass and polypropylene resulted in higher yields of hydrogen. It was, therefore, suggested that interaction was confined to gas phase reforming and cracking reactions which would not be evident from thermogravimetric analysis which only measures feedstock weight loss, reflecting the loss of volatiles.
Comparison of Ni/MCM-41 and Mo/MCM-41 catalysts for the co-pyrolysis–catalytic steam reforming of biopolymers and plastics
Initial experiments involved an investigation of a nickel-based MCM-41 catalyst in comparison to a molybdenum-based MCM-41 catalyst for the co-pyrolysis of the biopolymers (cellulose (Cell) and lignin (Lig)) and waste plastics (HDPE and PS) mixtures. The catalyst temperature was 750 °C and the steam:feedstock ratio was 2.85. The results for cellulose mixed with polyethylene and polystyrene are shown in Table 2 and the yield of the different product gas components is shown in Fig. 3. Table 3 and Fig. 4 show the results for lignin mixed with the different plastics in relation to the different catalysts. The total gas yield in terms of biopolymer/plastics only was 112.49 wt.% for cellulose/polyethylene and 119.22 wt.% for cellulose/polystyrene for the nickel catalyst but the total gas yield was lower for the molybdenum-based catalyst at 107.54 wt.% and 115.35 wt.%, respectively. The yields are over 100% for these results because the data are expressed in terms of the feedstock only and do not include the input of reacted water. The carbonaceous coke formation on the catalyst was reduced for the Mo/MCM-41 catalyst for the cellulose/polyethylene feedstock, but for the cellulose/polystyrene feedstock, there was little effect on catalyst coke formation. When the biopolymer, lignin, was co-pyrolysed with the waste plastics, the effect on the total gas yield was a marked reduction with the Mo/MCM-41 catalyst. But, the catalyst coke formation was significantly increased for the Mo/MCM-41 catalyst compared to the Ni/MCM-41 catalyst.
Figure 3 shows that the hydrogen yield from the Ni/MCM-41 catalyst was approximately 50.5 mmol g−1 for both the co-pyrolysis of cellulose with polyethylene and with polystyrene. The Mo/MCM-41 catalysts produced 46.5 mmol g−1 of hydrogen for co-pyrolysis catalytic steam reforming of cellulose/polyethylene and 48.0 mmol g−1 of hydrogen was produced from cellulose/polystyrene. However, Fig. 4 shows that the yield of hydrogen for the lignin mixtures was significantly lower for both the Ni/MCM-41 and Mo/MCM-41 catalysts compared to when cellulose was used in the co-pyrolysis mixture. The yield of hydrogen with the Ni/MCM-41 catalyst was 25.5 mmol g−1 for both the lignin/polyethylene and lignin/polystyrene feedstocks but was much lower for the Mo/MCM-41 catalyst at 16.0 mmol g−1 and 15.5 mmol g−1, respectively.
MCM-41 support material used in this work has a large surface area of about 1000 m2 g−1 and pore size distribution ranging from 2 to 10 nm. Due to the porous structure of the MCM-41 material, the interaction between the active Ni metal and MCM-41 support enhances the catalytic performance of the steam reforming of hydrocarbons [39]. For example, Zhao et al. [40] investigated the pyrolysis/catalytic decomposition of cellulose using TGA-mass spectrometry and reported a higher yield of hydrogen for a Ni/MCM-41 catalyst compared to a Ni/A2O3 catalyst which was attributed to the large surface area and pore size of the MCM-41 catalyst. It has also been suggested that the high surface area and uniform pore distribution of MCM-41 facilitates an increase in metal dispersion within the framework of the support, thereby enhancing catalyst activity [41].
The Ni/MCM-41 catalyst gave higher yields of hydrogen than the Mo/MCM-41 catalyst for the different cellulose/plastic mixtures and the influence on the deposition of carbonaceous coke on the catalyst was similar for the two catalysts, and in some cases catalyst coke deposition increased. Therefore, for the investigation of the influence of different process parameters on the pyrolysis–catalytic steam reforming of the biopolymer/plastic mixtures, the Ni/MCM-41 catalyst was used throughout.
Influence of catalyst temperature on the co-pyrolysis catalytic steam reforming of biopolymers and plastics
The influence of catalyst temperature at 750 °C and 850 °C was investigated in the two-stage reactor system for the co-pyrolysis of biopolymer and plastic mixtures followed by catalytic steam reforming of the pyrolysis gases. The pyrolysis of the feedstock was carried out by heating the feedstock at 20 °C min−1 to the final pyrolysis temperature of 550 °C, the catalyst used was 10 wt.% Ni/MCM-41 and the steam input was 5.7 mL h−1 representing a steam:feedstock ratio of 2.85. The product yield and gas composition as vol.% are shown in Table 4 and gas yield data as mmol g−1 are shown in Fig. 5. The total gas yield with respect to the feedstock (biopolymer/plastic) only showed that the gas yields in some cases were greater than 100 wt.%, particularly at the higher catalyst temperature because the reforming gas (steam) contributed to the calculated total gas yield but was not considered as input to the mass balance.
Table 4 shows that the gas yield increased for all of the biopolymer/plastic mixtures as the catalytic steam reforming temperature was increased from 750 °C to 850 °C. However, raising the catalyst steam reforming temperature by 100 °C for the cellulose/plastic mixtures produced an increase in gas yield from 112.49 to 125.24 wt.% for cellulose/HDPE and from 119.22 to 125.34 wt.% for cellulose/PS, representing an increase in the yield of only about 6‒13 wt.%. But for the lignin/plastic mixtures, the effect of increased catalytic reforming temperature produced a significantly increased gas yield from 70.85 to 107.09 wt.% for lignin/HDPE and from 65.20 to 102.51 wt.% for lignin/PS representing a far higher proportional increase of ~ 36 to 37 wt.% in gas yield compared with the cellulose/plastic mixtures. Therefore, the influence of temperature on gas yield was greater for lignin compared to cellulose, suggesting greater interaction of the lignin with the plastics. The char yields were higher for the mixtures containing lignin since lignin contains a significantly higher fixed carbon content (Table 1) compared to cellulose. The syngas (H2 and CO) yield was significantly higher for the cellulose/plastic mixtures compared to the lignin/plastic mixtures. Table 4 shows that the composition of the gas produced from co-pyrolysis–catalytic steam reforming contained a high proportion of hydrogen gas of ~ 60 vol.%, with the other main gas being carbon monoxide, indicating the product gas could be used as a high calorific value fuel or even a chemical feedstock.
Figure 5 shows that the influence of raising the catalyst temperature from 750 °C to 850 °C shows that the impact on the hydrogen yield was more pronounced for the biopolymer mixtures containing lignin compared to cellulose. The hydrogen yield for cellulose/HDPE (50.3 mmol g−1) and cellulose/PS (51.0 mmol g−1) showed an increase in hydrogen yield of ~ 10 mmol g−1 and ~ 7 mmol g−1, respectively, when the catalyst temperature was raised from 750 °C to 850 °C. However, for the lignin/HDPE mixture, the increase in hydrogen yield was from 25.0 mmol g−1 to 50.0 mmol g−1 representing an increase in hydrogen yield of 25 mmol g−1 as the catalyst temperature was raised from 750 °C to 850 °C. Similarly, for the lignin/PS mixture, the yield of hydrogen increased from 25.0 to 46.0 mmol g−1 representing an increase of 21 mmol g−1 of hydrogen.
The thermal decomposition of the mixture of cellulose/lignin and the polyethylene/polystyrene in the first stage pyrolysis reactor will produce a wide range of polymer fragments including oxygenated (CxHyOz) and non-oxygenated hydrocarbons (CnHm), as well as gases including H2, CH4 and CO, CO2 and H2O. The biopolymer decomposition will also produce lower amounts of CnHm hydrocarbons which will undergo cracking. The oxygenated hydrocarbons (CxHyOz) from the biopolymer decomposition and non-oxygenated hydrocarbons (CnHm) from the plastics decomposition will then undergo thermal cracking reactions in the hot zone of the second stage catalytic reactor (Eqs. 1 and 2, respectively).
Oxygenated hydrocarbons cracking:
Hydrocarbon cracking:
The oxygenated hydrocarbons (CxHyOz) and non-oxygenated hydrocarbons (CnHm) from the pyrolysis products will also undergo catalytic steam reforming (Eqs. 3 and 4).
Oxygenated hydrocarbons steam reforming:
Hydrocarbon steam reforming:
In addition, a range of other reactions may occur in the catalytic steam reforming reactor. For example, the production of CO2 from the pyrolysis of the biopolymers may induce catalytic dry (CO2) reforming of the oxygenated and non-oxygenated hydrocarbons (Eqs. 5 and 6).
Oxygenated hydrocarbons dry (CO2) reforming:
Hydrocarbon dry (CO2) reforming:
Also, the production of CO may promote the water gas shift reaction and Boudouard reaction (Eqs. 7 and 8, respectively)
Water gas shift reaction:
Boudouard reaction:
Chai et al. [10] investigated the pyrolysis–catalytic steam reforming of biomass (pine wood sawdust) and plastic (low-density polyethylene) and showed a similar increase in hydrogen yield with increasing catalytic steam reforming temperature. They used a two-stage, fixed bed pyrolysis–catalytic steam reforming reactor with a Ni–CaO–C catalyst. The catalyst was prepared with CaO to enable adsorption of product CO2 and thereby enhance hydrogen production. Raising the catalyst temperature from 500 °C to 600 °C produced a marked increase in H2 yield from 13.3 mmol g−1 to 115.3 mmol g−1 in terms of the biomass/plastic mixture. Further increasing the catalyst reforming temperature to 700 °C produced only a small increase in hydrogen yield.
The greater influence on total gas yield and hydrogen and syngas yield for the lignin/plastic mixtures compared to the cellulose/plastic mixtures is probably due to the overlapping thermal degradation profiles of lignin and the polyethylene and polystyrene (Fig. 2). However, cellulose had a peak degradation temperature of 350 °C, and much of the cellulose would be pyrolysed and the gases exited from the reactor before any interaction with the gases produced from the pyrolysis of the plastics occurred. Ahmed et al. [14] also suggested that the interaction of different feedstock materials can occur through volatile–volatile interaction or through volatile–fixed carbon interaction or both. Also, the plasticity of the certain feedstock materials may affect the evolution of volatiles of other feedstock. They conclude that materials having similar overlapping decomposition temperature ranges are likely to interact more. Burra and Gupta [38] also report that the overlap of thermal decomposition temperature regimes is necessary for the synergistic interaction between biomass and plastics. It has been suggested that interaction between volatiles and fixed carbon involves radical donor from the biomass degradation initiating and enhancing plastic polymer chain scission [17, 38, 42]. Lopez et al. [13] found a synergistic effect between biomass and high-density polyethylene, where the feedstock was co-fed into a fluidised spouted bed reactor followed by catalytic steam reforming in a separate fluidised bed reactor. The novel system was developed for the production of hydrogen-rich gas. They reported that char and hydrocarbon tar yields were reduced and gas yield increased to a greater extent than would be predicted based on individual processing. The co-feeding and fast pyrolysis would promote the interaction of volatiles and overcome the issue of the need for overlapping of the thermal degradation profiles for synergistic effects to be observed. Whereas in slow pyrolysis, fixed bed reactor experimental systems, less synergistic interaction would be expected for feedstocks with different thermal degradation profiles. For example, Lopez et al. [13] suggest that the char produced from biomass pyrolysis and retained in their fluidised spouted bed reactor for extended periods provides a reaction environment that favours hydrocarbon cracking, enhancing gas yield.
Other researchers have reported an increase in hydrogen production from biomass by the addition of HDPE. For example, Arregi et al. [16] investigated the co-pyrolysis–catalytic steam reforming of biomass (pine wood sawdust) and polyethylene (high-density polyethylene) in a novel two-stage spouted bed pyrolysis-fluidised bed catalytic steam reforming reactor. The catalyst used was a commercial Ni-catalyst. They reported a linear threefold increase in total product gas and hydrogen yield with the increasing amount of HDPE added to the feedstock. They also reported a reduction in catalyst deactivation with increased input of polyethylene in the biomass/plastic feedstock. This was attributed to the reduced formation of amorphous type carbon which is detrimental to catalytic activity and higher formation of filamentous type carbon which has a lower impact on activity [25]. Xu et al. [42] also used a two-stage reactor system with fixed bed fast pyrolysis of a biomass/plastic mixture (rice husks/polyethylene) followed by fixed bed catalytic reactor with a Ni/Al2O3 catalyst. Pyrolysis–catalytic steam reforming experiments showed an optimum biomass/polyethylene ratio of 1:1 which resulted in maximum H2 and CO yield attributed to steam reforming and water gas shift reactions. Alvarez et al. [12] investigated co-pyrolysis of biomass (waste wood sawdust) with different plastics and catalytic steam reforming of the pyrolysis gases in a two-stage, fixed bed reactor system. They also reported an increase in total gas yield and hydrogen yield with the addition of the plastics to the biomass of the different plastics investigated, polystyrene produced the lowest hydrogen yield compared to polypropylene, polyethylene, and mixed plastics. The aromatic nature of the pyrolysis products derived from polystyrene would require higher reaction energy for cracking and reforming.
Effect of different steam input on the co-pyrolysis–catalytic steam reforming of cellulose and plastics
Since cellulose/plastic mixtures produced significantly higher yields of hydrogen compared to lignin/plastic mixtures, experiments were undertaken to determine the influence of steam flow rate in relation to product yield and gas composition for the co-pyrolysis of cellulose/plastics (HDPE and PS) mixtures for the pyrolysis, catalytic steam reforming process. The catalyst used was the 10 wt.% Ni/MCM-41 catalyst. The catalytic bed temperature was kept constant at 850 °C for all the experiments conducted with a different steam flow rate. The co-pyrolysis was 1:1 ratio of biomass components with plastics waste at different steam flow rates from 0, 3.7, 5.7, 7.7, and 9.7 mL h−1 representing steam:feedstock ratios of 0, 1.85, 2.85, 3.85 and 4.85. The product yield and gas composition as vol.% and mmol g−1 biomass/plastic are shown in Table 5 and Fig. 6. The total gas yield was calculated based on Eqs. (1) and (2). The results for cellulose/HDPE showed that the increase in steam:feedstock ratio from 0 to 4.85 had a significant influence on the product yields. For the gas yield in relation to cellulose/HDPE feedstock only (Table 5), there was a consistent increase in the total gas yield from 59.40 to 129.48 wt.%, for the cellulose/HDPE mixture as the steam:feedstock ratio was increased from 0 to 3.85. There was then a slight decrease to 124.92 wt.% as the steam:feedstock ratio was raised further to 4.85. In relation to the cellulose/PS mixture, the gas yield increased from 57.64 to 147.45 wt.% as the steam input was increased to give a steam:feedstock ratio of 3.85 and then decreased to 142.17 wt.% as steam flow rate was increased to produce a steam:feedstock ratio of 4.85. The results show that the influence of increased steam input to the process was greater for the lignin/plastic mixtures compared to the cellulose/plastic mixtures. Suggesting that there is greater interaction between lignin and the waste plastics compared to the cellulose/plastic mixtures.
The higher gas yield with the increase in steam input was due to the increased promotion of the catalytic steam reforming of hydrocarbons, oxygenated hydrocarbons, and aliphatic hydrocarbons produced from the co-pyrolysis of cellulose and plastics. In addition, the increased gas yield will result from thermal and catalytic cracking of these hydrocarbons, dry (CO2) reforming, water gas shift reaction, char gasification, etc. The two-stage pyrolysis–catalytic steam reforming reactor system has been used to effectively produce high-yield hydrogen syngas. The two-stage reaction system has advantages over a single-stage reactor where the biomass and catalyst are mixed together, in that there is more effective separate control of the process conditions of the pyrolysis and catalyst stages, e.g. temperature, steam input, etc.[43].
The gas yield (mmol g−1) in terms of H2, CH4 C2H4, CO, and CO2 in relation to steam input into the catalytic steam reforming reactor is shown in Fig. 6, the syngas yield in mmol g−1 is shown in Table 5. The yield of hydrogen and syngas was positively enhanced with an increase in the steam flow rate. For example, in the absence of steam input into the co-pyrolysis–catalytic steam reforming process, the hydrogen yield was 32.0 mmol g−1 for the cellulose/HDPE feedstock and 29.5 mmol g−1 for the HDPE/PS feedstock. The production of hydrogen in the absence of steam is suggested to be through the thermal and catalytic cracking of the pyrolysis gases. As steam was introduced into the process, the hydrogen yield showed a marked increase to more than 60 mmol g−1 at the higher steam inputs. At the highest steam:feedstock ratio of 4.85 there was a decrease in hydrogen yield for the cellulose/PS but for the cellulose/HDPE there was a slight increase. Higher steam inputs that were used in the two-stage pyrolysis catalytic steam reforming process compared to those used in this work have shown a significant decrease in hydrogen yield [43]. The syngas yield (H2 and CO) (Table 5) showed that there was an initial increase in syngas yield as the steam input was increased, but at the highest steam input, the syngas yield showed a slight decrease. The increase in steam flow rate during reforming introduces extra oxygen and hydrogen into the system. At high temperatures such as 850 °C investigated in this study, the water gas shift reaction is enhanced as steam input increases, therefore, accounting for the increase in H2 and CO up to a steam:feedstock ratio of 3.85 but decreases slightly as the steam:feedstock ratio is increased to 4.85. CH4 yield decreases while C2–C4 is negligible at increasing steam input rate.
Chai et al. [10] used a two-stage fixed bed pyrolysis–catalytic steam reforming reactor system with a biomass/plastic feedstock in the form of pine sawdust/low-density polyethylene. They reported an initial increase in hydrogen yield as the steam flow rate was increased but decreased at higher steam inputs. Ruoppolo et al. [44] also investigated the effect of steam for the co-pyrolysis of biomass and plastic and showed that there was an increase in hydrogen yield and H2/CO and CO2/CO ratios and a reduction in tar concentration suggesting that the presence of steam favoured the water gas shift reactions and reforming of hydrocarbons. Li et al. [45] also showed an increase in hydrogen yield with increasing steam input but a decrease at higher inputs. They suggested that the initial increase in hydrogen yield was because of the water gas and water gas shift reactions but at higher steam inputs, the excess steam flowing through the reactor system produced a decrease in temperature because of the endothermic reaction of steam generation and suppressed reaction. However, it has also been suggested that the surface of the catalyst becomes saturated by steam reforming in the reaction system [43].
Effect of Ni-catalyst support material on the co-pyrolysis–catalytic steam reforming of cellulose and plastics
The production of hydrogen from cellulose/plastic mixtures in relation to the Ni-MCM-41 catalyst was further investigated to compare different catalyst support materials. The results obtained with the Ni/MCM-41 catalyst were compared with those obtained from Ni/Al2O3 and Ni/Y-zeolite. The catalytic bed temperature was kept constant at 850 °C for all the experiments and the steam:biomass ratio was maintained at 2.85. Nickel/Al2O3 catalysts have been used to produce hydrogen from waste plastics since this type of catalyst is commonly used for the commercial production of hydrogen from natural gas [9, 19]. In addition, Al2O3 is known to be chemically and physically stable with high mechanical strength properties [24]. Ni/zeolite catalysts, particularly Ni-ZSM-5 type catalysts have been investigated for the production of hydrogen from waste plastics [21, 46, 47] but fewer data are available for Ni/Y-zeolite which has a larger pore size and surface area than ZSM-5 catalysts. The introduction of nickel into zeolites has been suggested to influence the surface acidity which in turn influences coke formation [46] and the mesoporosity and microporosity of zeolite also improve the dispersion of the nickel particles within the pores of the support [21, 24]. Table 6 shows the product yield for different nickel catalyst support material, Ni/MCM-41, Ni/Al2O3, and Ni/Y-zeolite, in relation to the co-pyrolysis steam reforming of cellulose and plastics waste blends. The total gas yield in relation to the biomass and plastics only was significantly higher for the Ni/Al2O3 catalyst compared to the N-MCM-41 and Ni/Y-zeolite catalysts. The high yield of gas was reflected in the higher production of syngas (H2 and CO) for the Ni/Al2O3 catalyst for both the cellulose/HDPE and cellulose/polystyrene mixtures at 102.01 mmol g−1 and 100.13 mmol g−1, respectively. It is also worthy to note that the deposition of carbonaceous coke on the Ni/Al2O3 catalyst was not detected, whereas the more porous MCM-41 and Y-zeolite catalysts showed carbon deposits. The volumetric gas composition was quite similar for the three Ni-supported catalysts investigated, even though the gas yields were different. Figure 7 shows the component gas yield in relation to the Ni/MCM-41, Ni/Al2O3, and Ni/Y-zeolite catalysts for the different cellulose/plastic mixtures. The hydrogen and carbon monoxide yields produced with the Ni/Al2O3 catalyst were higher than the other two catalysts suggesting a more effective catalytic steam reforming of the pyrolysis gases.
The influence of the catalyst support material is clearly an important process variable that influences hydrogen and syngas yield. This study set out to investigate the role of MCM-41 as a viable support material for the production of hydrogen from the co-pyrolysis of biopolymers (cellulose and lignin) and plastics (polyethylene and polystyrene). From the work reported here, it appears that MCM-41 is less effective than the more commonly used Al2O3 support material.
Overall, the results have demonstrated that co-pyrolysis of waste biomass and plastics coupled with catalytic steam reforming of the evolved pyrolysis gases can produce significant yields of hydrogen and syngas. The particular biopolymers investigated (cellulose and lignin) showed that cellulose produces significantly higher yields of hydrogen and syngas and also the total gas yield was higher compared with lignin mixtures with plastics. Consequently, biomass types with high lignin content are likely to produce less hydrogen compared to biomass types which are high in cellulose content when mixed with waste plastics. Municipal solid waste (MSW) contains a mixture of biomass and plastics along with non-organic metals and glass. The fraction of biomass and plastics may be increased by processing to produce refuse-derived fuels. The main types of plastics in MSW are high-density polyethylene, low-density polyethylene, polypropylene, polystyrene, polyvinyl chloride, and polyethylene terephthalate (PET) [9]. The biomass components of MSW and thereby refuse-derived fuel include several different biomass materials, such as paper, cardboard, wood, food waste, etc. A high proportion of paper would increase the cellulose content of the final product. The results reported here, therefore, also have relevance to the processing of organic feedstocks produced from MSW where the cellulose content could be manipulated to maximise the production of hydrogen.
Conclusions
The co-pyrolysis–catalytic steam reforming of biomass components (lignin and cellulose) with plastic wastes (polyethylene and polystyrene) using a Ni/MCM-41 catalyst in a fixed bed two-stage reactor have been investigated. Nickel supported on MCM-41 catalyst produced a higher yield of hydrogen compared to Mo/MCM-41. The cellulose/plastic mixtures produced significantly a higher yield of total gas, syngas and hydrogen compared to the lignin/plastic mixtures. However, the increase in catalytic steam reforming temperature in relation to total gas yield, syngas, and hydrogen yield was much more pronounced for the lignin/plastic mixtures compared to the cellulose/lignin mixtures, suggesting an interaction between the thermal decomposition of lignin and the waste plastics.
The introduction of steam to the co-pyrolysis, catalytic steam reforming process produced a marked increase in total gas yield, syngas, and hydrogen yields. The yield of hydrogen and syngas was optimised at a steam injection with a steam/feedstock ratio of 3.85 and catalytic steam reforming temperature of 850 °C. Comparison of the MCM-41 support material with Al2O3 and Y-zeolite support materials showed that the most effective catalyst in terms of hydrogen and syngas yield was the more commonly used Ni/Al2O3 catalyst.
References
IEA. The Future of hydrogen: seizing todays opportunities. In: Report prepared by the IEA for the G20, Japan. International Energy Agency, Paris, 2019.
Dincer I, Acar C. Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrogen Energy. 2015;40:11094–111.
Balat H, Kırtay E. Hydrogen from biomass–present scenario and future prospects. Int J Hydrogen Energy. 2010;35(14):7416–26.
Kirkels AF, Verbong GP. Biomass gasification: Still promising? A 30-year global overview. Renew Sustain Energy Rev. 2011;15(1):471–81.
Akubo K, Nahil MA, Williams PT. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J Energy Inst. 2019;92(6):1987–96.
Blanco PH, Wu C, Williams PT. Influence of Ni/SiO2 catalyst preparation methods on hydrogen production from the pyrolysis/reforming of refuse derived fuel. Int J Hydrogen Energy. 2014;39:5723–32.
Lopez G, Artetxe M, Amutio M, et al. Recent advances in the gasification of waste plastics. A critical overview. Renew Sustain Energy Rev. 2018;82:576–96.
Barbarias I, Lopez G, Alvarez J, et al. Valorisation of different waste plastics by pyrolysis and in-line catalytic steam reforming for hydrogen production. Energy Convers Manag. 2018;156:575–84.
Williams PT. Hydrogen & carbon nanotubes from pyrolysis-catalysis of waste plastics: a review. Waste Biomass Valorization. 2020. https://doi.org/10.1007/s12649-020-01054-w.
Chai Y, Gao N, Wang M, et al. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem Eng J. 2020;382:122947.
Liu WW, Hu CW, Yang Y, et al. Study on the effect of metal types in (Me)-Al-MCM-41 on the mesoporous structure and catalytic behavior during the vapor-catalyzed co-pyrolysis of pubescens and LDPE. Appl Catal B. 2013;129:202–13.
Alvarez J, Kumagai S, Wu C, et al. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int J Hydrogen Energy. 2014;39(21):10883–91.
Lopez G, Erkiaga A, Amutio M, et al. Effect of polyethylene co-feeding in the steam gasification of biomass in a conical spouted bed reactor. Fuel. 2015;153:393–401.
Ahmed I, Nipattummakul N, Gupta A. Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl Energy. 2011;88(1):165–74.
Pinto F, Franco C, André R, et al. Co-gasification study of biomass mixed with plastic wastes. Fuel. 2002;81(3):291–7.
Arregi A, Amutio M, Lopez G, et al. Hydrogen-rich gas production by continuous pyrolysis and in-line catalytic reforming of pine wood waste and HDPE mixtures. Energy Conserv Manag. 2017;136:192–201.
Block C, Ephraim A, Weiss-Hortala E, et al. C0-pyrogasification of plastics and biomass. Waste Biomass Valorization. 2019;10(3):483–509.
Dong L, Wu C, Ling H, et al. Promoting hydrogen production and minimizing catalyst deactivation from the pyrolysis-catalytic steam reforming of biomass on nanosized NiZnAlOx catalysts. Fuel. 2017;188:610–20.
Czernik S, French RJ. Production of hydrogen from plastics by pyrolysis and catalytic steam reform. Energy Fuels. 2006;20:754–8.
Namioka T, Saito A, Inoue Y, et al. Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst. Appl Energy. 2011;88:2019–26.
Yao D, Yang H, Chen H, et al. Investigation of nickel impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene. Appl Catal B Environ. 2018;227:477–87.
Wu C, Williams PT. Nickel-based catalysts for tar reduction in biomass gasification. Biofuels. 2011;2:451–64.
Wu C, Williams PT. Investigation of Ni-Al, Ni-Mg-Al and Ni-Cu-Al catalysts for hydrogen production from pyrolysis-gasification of polypropylene. Appl Catal B. 2009;90:147–56.
Ochoa A, Bilbao J, Gayubo AG, et al. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products; a review. Renew Sustain Energy Rev. 2020;119:109600.
Wu C, Williams PT. Investigation of coke formation on Ni-Mg-Al catalyst for hydrogen production from the catalytic steam pyrolysis-gasification of polypropylene. Appl Catal B Environ. 2010;96:198–207.
Claridge JB, York APE, Brungs AJ, et al. New catalysts for the conversion of methane to synthesis gas: molybdenum and tungsten carbide. J Catal. 1998;180:85–100.
Ma Y, Guan G, Hao X, et al. Molybdenum carbide as alternative catalyst for hydrogen production—a review. Renew Sustain Energy Rev. 2017;75:1101–29.
Liu S, Chen M, Chu L, et al. Catalytic steam reforming of bio-oil aqueous fraction for hydrogen production over Ni-Mo supported on modified sepiolite catalysts. Int J Energy Res. 2013;38:3948–55.
Figen HE, Baykara SZ. Effect of ruthenium addition on molybdenum catalysts for syngas production via catalytic partial oxidation of methane in a monolithic reactor. Int J Energy Res. 2018;43:1129–38.
Zhang Y, Huang J, Williams PT. Fe-Ni-MCM-41 catalysts for hydrogen-rich syngas production from waste plastics by pyrolysis-catalytic steam reforming. Energy Fuels. 2017;32(8):8497–504.
Wu C, Wang LZ, Williams PT, et al. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: influence of Ni content. Appl Catal B. 2011;108:6–13.
Zhang X, Lei H, Chen S, et al. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: a critical review. Green Chem. 2016;18(15):4145–69.
Giudicianni P, Cardone G, Ragucci R. Cellulose, hemicellulose and lignin slow steam pyrolysis: thermal decomposition of biomass components mixtures. J Anal Appl Pyrol. 2013;100:213–22.
Yang H, Yan R, Chen H, et al. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuels. 2006;20(1):388–93.
Jin W, Shen D, Liu Q, et al. Evaluation of the co-pyrolysis of lignin with plastic polymers by TG-FTIR and Py-GC/MS. Polym Degrad Stab. 2016;133:65–74.
Oyedun AO, Gebreegzaibher T, Ng DKS, et al. Mixed waste pyrolysis of biomass and plastics waste—a modelling approach to reduce energy usage. Energy. 2014;75:127–35.
Sharypov VI, Beregovtsova NG, Baryshnikov SV, et al. Co-pyrolysis of wood biomass and synthetic polymer mixtures. Part 1: Influence of experimental conditions on the evolution of solids, liquids and gases. J Anal Appl Pyrolysis. 2002;64:15–28.
Burra KG, Gupta AK. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl Energy. 2018;211:230–6.
Wu C, Dong L, Onwudili J, et al. Effect of Ni particle location within the mesoporous MCM-41 support for hydrogen production from the catalytic gasification of biomass. ACS Sustain Chem Eng. 2013;1:1083–91.
Zhao M, Florin NH, Harris AT. The influence of supported Ni catalysts on the product gas distribution and H2 yield during cellulose pyrolysis. Appl Catal B-Environ. 2009;92(1–2):185–93.
Lucredio AF, Assaf EM. Cobalt catalysts prepared from hydrotalcite precursors and tested in methane steam reforming. J Power Sour. 2006;159(1):667–72.
Xu D, Xiong Y, Ye J, et al. Performances of syngas production and deposited coke regulation during co-gasification of biomass and plastic wastes over Ni/γ-Al2O3 catalyst: role of biomass to plastic ratio in feedstock. Chem Eng J. 2020;392:123728.
Wu C, Williams PT. Hydrogen production from the pyrolysis-gasification of polypropylene: influence of steam flow rate, carrier gas flow rate and gasification temperature. Energy Fuels. 2009;23:5055–61.
Ruoppolo G, Ammendola P, Chirone R, et al. H2-rich syngas production by fluidized bed gasification of biomass and plastic fuel. Waste Manag. 2012;32(4):724–32.
Li J, Yin Y, Zhang X, et al. Hydrogen-rich gas production by steam gasification of palm oil wastes over supported tri-metallic catalyst. Int J Hydrogen Energy. 2009;34(22):9108–15.
Wu C, Williams PT. Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl Catal B. 2009;87:152–61.
Yao W, Li J, Feng Y, Wang W, et al. Thermally stable phosphorus and nickel modified ZSM-5 zeolites for catalytic co-pyrolysis of biomass and plastics. RSC Adv. 2015;5(39):30485–94.
Acknowledgements
Financial support from the Petroleum Technology Development Fund (PTDF) of Nigeria for a scholarship for K.A. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akubo, K., Nahil, M.A. & Williams, P.T. Co-pyrolysis–catalytic steam reforming of cellulose/lignin with polyethylene/polystyrene for the production of hydrogen. Waste Dispos. Sustain. Energy 2, 177–191 (2020). https://doi.org/10.1007/s42768-020-00047-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-020-00047-8