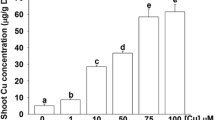
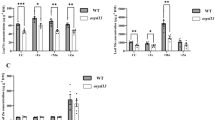
Key message
Rice aconitase gene OsACO1 is involved in the iron deficiency-signaling pathway for the expression of iron deficiency-inducible genes, either thorough enzyme activity or possible specific RNA binding for post-transcriptional regulation.
Abstract
Iron (Fe) is an essential element for virtually all living organisms. When plants are deficient in Fe, Fe acquisition systems are activated to maintain Fe homeostasis, and this regulation is mainly executed at the gene transcription level. Many molecules responsible for Fe uptake, translocation, and storage in plants have been identified and characterized. However, how plants sense Fe status within cells and then induce a transcriptional response is still unclear. In the present study, we found that knockdown of the OsACO1 gene, which encodes an aconitase in rice, leads to the down-regulation of selected Fe deficiency-inducible genes involved in Fe uptake and translocation in roots, and a decrease in Fe concentration in leaves, even when grown under Fe-sufficient conditions. OsACO1 knockdown plants showed a delayed transcriptional response to Fe deficiency compared to wild-type plants. In contrast, overexpression of OsACO1 resulted in the opposite effects. These results suggest that OsACO1 is situated upstream of the Fe deficiency-signaling pathway. Furthermore, we found that the OsACO1 protein potentially has RNA-binding activity. In vitro screening of RNA interactions with OsACO1 revealed that RNA potentially forms a unique stem-loop structure that interacts with OsACO1 via a conserved GGUGG motif within the loop structure. These results suggest that OsACO1 regulate Fe deficiency response either thorough enzyme activity catalyzing isomerization of citrate, or specific RNA binding for post-transcriptional regulation.





Similar content being viewed by others
References
Anderson CP, Shen M, Eisenstein RS, Leibold EA (2012) Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823:1468–1483
Armenteros JJA, Salvatore M, Emanuelsson O, Winther O, von Heijne G, Elofsson A, Nielsen H (2019) Detecting sequence signals in targeting peptides using deep learning. Life Sci Alli 2:e201900429
Arnaud N, Ravet K, Borlotti A, Touraine B, Boucherez J, Fizames C, Briat JF, Cellier F, Gaymard F (2007) The iron-responsive element (IRE)/iron-regulatory protein 1 (IRP1)-cytosolic aconitase iron-regulatory switch does not operate in plants. Biochem J 405:523–531
Aung MS, Kobayashi T, Masuda H, Nishizawa NK (2018) Rice HRZ ubiquitin ligases are crucial for response to excess iron. Physiol Plant 163:282–296
Balk J, Schaedler TA (2014) Iron cofactor assembly in plants. Ann Rev Plant Biol 65:125–153
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Bashir K, Ishimaru Y, Itai RN, Senoura T, Takahashi M, An G, Oikawa T, Ueda M, Sato A, Uozumi N, Nakanishi H, Nishizawa NK (2015) Iron deficiency regulated OsOPT7 is essential for iron homeostasis in rice. Plant Mol Biol 88:165–176
Bashir K, Ishimaru Y, Shimo H, Nagasaka S, Fujimoto M, Takanashi H, Tsutsumi N, An G, Nakanishi H, Nishizawa NK (2011) The rice mitochondrial iron transporter is essential for plant growth. Nat Commun 2:1–7
Bellis L, Hayashi M, Biagi PP, Hara-Nishimura I, Alpi A, Nishimura M (1994) Immunological analysis of aconitase in pumpkin cotyledons: the absence of aconitase in glyoxysomes. Physiol Plant 90:757–762
Bernard DG, Cheng Y, Zhao Y, Balk J (2009) An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron-sulfur proteins in Arabidopsis. Plant Physiol 151:590–602
Carrari F, Nunes-Nesi A, Gibon Y, Lytovchenko A, Loureiro ME, Fernie AR (2003) Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol 133:1322–1335
Courtois-Verniquet F, Douce R (1993) Lack of aconitase in glyoxysomes and peroxisomes. Biochem J 294:103–107
Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, Fontecilla-Camps JC (2006) Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure 14:129–139
Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144:197–205
Eanes RZ, Kun E (1971) Separation and characterization of aconitate hydratase isoenzymes from pig tissues. Biochim Biophys Acta 227:204–210
Grillet L, Lan P, Li W, Mokkapati G, Schmidt W (2018) IRON MAN is a ubiquitous family of peptides that control iron transport in plants. Nat Plants 4:953–963
Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104:815–820
Hentze MW, Kühn LC (1996) Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA 93:8175–8182
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Hindt MN, Guerinot ML (2012) Getting a sense for signals: regulation of the plant iron deficiency response. Biochim Biophys Acta 1823:1521–1530
Hindt MN, Akmakjian GZ, Pivarski KL, Punshon T, Baxter I, Salt DE, Guerinot ML (2017) BRUTUS and its paralogs, BTS LIKE1 and BTS LIKE2, encode important negative regulators of the iron deficiency response in Arabidopsis thaliana. Metallomics 9:876–890
Hirayama T, Lei GJ, Yamaji N, Nakagawa N, Ma JF (2018) The putative peptide gene FEP1 regulates iron deficiency response in Arabidopsis. Plant Cell Physiol 59:1739–1752
Huang S, Taylor NL, Narsai R, Eubel H, Whelan J, Millar AH (2009) Experimental analysis of the rice mitochondrial proteome, its biogenesis, and heterogeneity. Plant Physiol 149:719–734
Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2003) Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 36:366–381
Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) A rice FRD3-like (OsFRDL1) gene is expressed in the cells involved in long-distance transport. Soil Sci Plant Nutr 50:1133–1140
Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, Nishizawa NK (2008) Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol 66:193–203
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK (2009) Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 284:3470–3479
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346
Ishimaru Y, Bashir K, Fujimoto M, An G, Itai RN, Tsutsumi N, Nakanishi H, Nishizawa NK (2009) Rice-specific mitochondrial iron-regulated gene (MIR) plays an important role in iron homeostasis. Mol Plant 2:1059–1066
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for long distance transport of iron and manganese. Plant J 62:379–390
Itai RN, Ogo Y, Kobayashi T, Nakanishi H, Nishizawa NK (2013) Rice genes involved in phytosiderophore biosynthesis are synchronously regulated during the early stages of iron deficiency in roots. Rice 6:16
Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, An K, Han MJ, Sung RJ, Choi HS, Yu JH, Choi JH, Cho SY, Cha SS, Kim SI, An G (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22:561–570
Karimi M, Inzé D, Depicker A (2002) GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Kennedy MC, Mende-Mueller L, Blondin GA, Beinert H (1992) Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci USA 89:11730–11734
Klausner RD, Rouault TA, Harford JB (1993) Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19–28
Kobayashi T, Yoshihara T, Jiang T, Goto F, Nakanishi H, Mori S, Nishizawa NK (2003) Combined deficiency of iron and other divalent cations mitigates the symptoms of iron deficiency in tobacco plants. Physiol Plant 119:400–408
Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2005) Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot 56:1305–1316
Kobayashi T, Ogo Y, Itai RN, Nakanishi H, Takahashi M, Mori S, Nishizawa NK (2007) The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Natl Acad Sci USA 104:19150–19155
Kobayashi T, Ogo Y, Aung MS, Nozoye T, Itai RN, Nakanishi H, Yamakawa T, Nishizawa NK (2010) The spatial expression and regulation of transcription factors IDEF1 and IDEF2. Ann Bot 105:1109–1117
Kobayashi T, Itai RN, Aung MS, Senoura T, Nakanishi H, Nishizawa NK (2012) The rice transcription factor IDEF1 directly binds to iron and other divalent metals for sensing cellular iron status. Plant J 69:81–91
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kobayashi T, Nagasaka S, Senoura T, Itai RN, Nakanishi H, Nishizawa NK (2013) Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat Commun 4:2792
Kobayashi T, Nishizawa NK (2014) Iron sensors and signals in response to iron deficiency. Plant Sci 224:36–43
Kobayashi T (2019) Understanding the complexity of iron sensing and signaling cascades in plants. Plant Cell Physiol 60:1440–1446
Kobayashi T, Nozoye T, Nishizawa NK (2019a) Iron transport and its regulation in plants. Free Radic Biol Med 133:11–20
Kobayashi T, Ozu A, Kobayashi S, An G, Jeon JS, Nishizawa NK (2019b) OsbHLH058 and OsbHLH059 transcription factors positively regulate iron deficiency responses in rice. Plant Mol Biol 101:471–486
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424
Kourmpetis YA, Van Dijk AD, Bink MC, Van Ham RC, TerBraak CJ (2010) Bayesian markov random field analysis for protein function prediction based on network data. PLoS ONE 5:e9293
Kunze M, Pracharoenwattana I, Smith SM, Hartig A (2006) A central role for the peroxisomal membrane in glyoxylate cycle function. Biochim Biophys Acta 1763:1441–1452
Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G (2009) Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol 150:786–800
Liang G, Zhang H, Li Y, Pu M, Yang Y, Li C, Lu C, Xu P, Yu D (2020) Oryza sativa FER-LIKE FE DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (OsFIT/OsbHLH156) interacts with OsIRO2 to regulate iron homeostasis. J Integ Plant Biol. https://doi.org/10.1111/jipb.12933
Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Mühlenhoff U (2012) The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823:1491–1508
Ma J, Haldar S, Khan MA, Sharma SD, Merrick WC, Theil EC, Goss DJ (2012) Fe2+ binds iron responsive element-RNA, selectively changing protein-binding affinities and regulating mRNA repression and activation. Proc Natl Acad Sci USA 109:8417–8422
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Mendoza-Cózatl DG, Xie Q, Akmakjian GZ, Jobe TO, Patel A, Stacey MG, Song L, Demoin DW, Jurisson SS, Stacey G, Schroeder JI (2014) OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Mol Plant 7:1455–1469
Moeder W, del Pozo O, Navarre DA, Martin GB, Klessig DF (2007) Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol Biol 63:273–287
Moroishi T, Nishiyama M, Takeda Y, Iwai K, Nakayama KI (2011) The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab 14:339–351
Nishitani Y, Okutani H, Takeda Y, Uchida T, Iwai K, Ishimori K (2019) Specific heme binding to heme regulatory motifs in iron regulatory proteins and its functional significance. J Inorg Biochem 198:110726
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011) Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 286:5446–5454
Nozoye T, Nakanishi H, Nishizawa NK (2013) Characterizing the crucial components of iron homeostasis in the maize mutants ys1 and ys3. PLoS ONE 8:e62567
Nozoye T, Nagasaka S, Kobayashi T, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2015) The phytosiderophore efflux transporter TOM2 is involved in metal transport in rice. J Biol Chem 290:27688–27699
Nozoye T, von Wirén N, Sato Y, Higashiyama T, Nakanishi H, Nishizawa NK (2019) Characterization of the nicotianamine exporter ENA1 in rice. Front Plant Sci 10:502
Ogo Y, Itai RN, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa NK (2006) Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot 57:2867–2878
Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK (2007) The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377
Ogo Y, Kobayashi T, Itai RN, Nakanishi H, Kakei Y, Takahashi M, Toki S, Mori S, Nishizawa NK (2008) A novel NAC transcription factor IDEF2 that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol Chem 283:13407–13417
Ogura M, Endo R, Ishikawa H, Takeda Y, Uchida T, Iwai K, Kobayashi K, Ishimori K (2018) Redox-dependent axial ligand replacement and its functional significance in heme-bound iron regulatory proteins. J Inorg Biochem 182:238–248
Peyret P, Perez P, Alric M (1995) Structure, genomic organization, and expression of the Arabidopsis thaliana aconitase gene. Plant aconitase show significant homology with mammalian iron-responsive element-binding protein. J Biol Chem 270:8131–8137
Philpott CC, Klausner RD, Rouault TA (1994) The bifunctional iron-responsive element binding protein/cytosolic aconitase: the role of active-site residues in ligand binding and regulation. Proc Natl Acad Sci USA 91:7321–7325
Rey P, Taupin-Broggini M, Couturier J, Vignols F, Rouhier N (2019) Is there a role for glutaredoxins and BOLAs in the perception of the cellular iron status in plants? Front Plant Sci 10:712
Rodríguez-Celma J, Chou H, Kobayashi T, Long TA, Balk J (2019a) Hemerythrin E3 ubiquitin ligases as negative regulators of iron homeostasis in plants. Front Plant Sci 10:98
Rodríguez-Celma J, Connorton JM, Kruse I, Green RT, Franceschetti M, Chen YT, Cui Y, Ling HQ, Yeh KC, Balk J (2019b) Arabidopsis BRUTUS-LIKE E3 ligases negatively regulate iron uptake by targeting transcription factor FIT for recycling. Proc Natl Acad Sci 116:17584–17591
Rouault TA (2009) An ancient gauge for iron. Science 326:676–677
Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK (2009) An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 326:722–726
Sato Y, Antonio BA, Namiki N, Takehisa H, Minami H, Kamatsuki K, Sugimoto K, Shimizu Y, Hirochika H, Nagamura Y (2011) RiceXPro: a platform for monitoring gene expression in japonica rice grown under natural field conditions. Nuc Acids Res 39:D1141–1148
Sato Y, Takehisa H, Kamatsuki K, Minami H, Namiki N, Ikawa H, Ohyanagi H, Sugimoto K, Antonio B, Nagamura Y (2013) RiceXPro Version 3.0: expanding the informatics resource for rice transcriptome. Nuc Acids Res 41:D1206–D1213
Selote D, Samira R, Matthiadis A, Gillikin JW, Long TA (2015) Iron-binding E3 ligase mediates iron response in plants by targeting basic helix-loop-helix transcription factors. Plant Physiol 167:273–286
Stacey MG, Patel A, McClain WE, Mathieu M, Remley M, Rogers EE, Gassmann W, Blevins DG, Stacey G (2008) The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol 146:589–601
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003) Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15:1263–1280
Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62:4843–4850
Urzica EI, Casero D, Yamasaki H, Hsieh SI, Adler LN, Karpowicz SJ, Blaby-Haas CE, Clarke SG, Loo JA, Pellegrini M, Merchant SS (2012) Systems and trans-system level analysis identifies conserved iron deficiency responses in the plant lineage. Plant Cell 24:3921–3948
Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, Spruck C, Leibold EA, Wohlschlegel JA (2009) Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326:718–721
Vigani G, Pii Y, Celletti S, Maver M, Mimmo T, Cesco S, Astolfi S (2018) Mitochondria dysfunctions under Fe and S deficiency: is citric acid involved in the regulation of adaptive responses? Plant Physiol Biochem 126:86–96
Wallace A, Lunt OR (1960) Iron chlorosis in horticultural plants, a review. Proc Am Soc Hort Sci 75:819–840
Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, Volz K (2006) Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 314:1903–1908
Walden WE, Selezneva A, Volz K (2012) Accommodating variety in iron-responsive elements: Crystal structure of transferrin receptor 1 B IRE bound to iron regulatory protein 1. FEBS Lett 586:32–35
Wallander ML, Leibold EA, Eisenstein RS (2006) Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta 1763:668–689
Wang F, Itai RN, Nozoye T, Kobayashi T, Nishizawa NK, Nakanishi H (2020a) The bHLH protein OsIRO3 is critical for plant survival and iron (Fe) homeostasis in rice (Oryza sativa L.) under Fe-deficient conditions. Soil Sci Plant Nutr in press. https://doi.org/10.1080/00380768.2020.1783966
Wang S, Li L, Ying Y, Wang J, Shao JF, Yamaji N, Whelan J, Ma JF, Shou H (2020b) A transcription factor OsbHLH156 regulates strategy II iron acquisition through localizing IRO2 to the nucleus in rice. New Phytol 225:1247–1260
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149:297–305
Yokosho K, Yamaji N, Ma JF (2016) OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J Exp Bot 67:5485–5494
Zhai Z, Gayomba SR, Jung HI, Vimalakumari NK, Piñeros M, Craft E, Rutzke MA, Danku J, Lahner B, Punshon T, Guerinot ML, Salt DE, Kochian LV, Vatamaniuk OK (2014) OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 26:2249–2264
Zhang Y, Xu YH, Yi HY, Gong JM (2012) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72:400–410
Zhang H, Li Y, Yao X, Liang G, Yu D (2017) POSITIVE REGULATOR OF IRON HOMEOSTASIS1, OsPRI1, facilitates iron homeostasis. Plant Physiol 175:543–554
Zhang H, Li Y, Pu M, Xu P, Liang G, Yu D (2020) Oryza sativa POSITIVE REGULATOR OF IRON DEFICIENCY RESPONSE 2 (OsPRI2) and OsPRI3 are involved in the maintenance of Fe homeostasis. Plant Cell Environ 43:261–274
Zheng L, Ying Y, Wang L, Wang F, Whelan J, Shou H (2010) Identification of a novel iron regulated basic helix-loop-helix protein involved in Fe homeostasis in Oryza sativa. BMC Plant Biol 10:166
Zuchi S, Watanabe M, Hubberten HM, Bromke M, Osorio S, Fernie AR, Celletti S, Paolacci AR, Catarcione G, Ciaffi M, Hoefgen R, Astolfi S (2015) The interplay between sulfur and iron nutrition in tomato. Plant Physiol 169:2624–2639
Zuo J, Wu Z, Li Y, Shen Z, Feng X, Zhang M, Ye H (2017) Mitochondrial ABC transporter ATM3 is essential for cytosolic iron-sulfur cluster assembly. Plant Physiol 173:2096–2109
Acknowledgements
We thank Dr. Yoshiaki Nagamura and Ms. Rituko Motoyama (National Institute of Agrobiological Sciences, Japan) for the rice microarray analysis, Ms. Akane Konishi, Ms. May Linn Aung, Ms. Hiroko Hori, and Ms. Mariko Sakashita (Ishikawa Prefectural University, Japan) for assistance with the rice culture and analysis. This research was supported by Advanced Low Carbon Technology Research and Development Program (ALCA) from Japan Science and Technology Agency (JST) (to N. K. N), and the Japan Society for the Promotion of Sciences (JSPS) KAKENHI Grant Numbers 16H04891 (to N. K. N) and 18H02115 (to T. K.).
Author information
Authors and Affiliations
Contributions
TS designed the research, carried out most of the experiments, and wrote the manuscript with discussion from all the co-authors. TK carried out data analysis and manuscript revision. GA provided the aco1(+ /−) T-DNA lines. HN and NKN supervised the research.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Senoura, T., Kobayashi, T., An, G. et al. Defects in the rice aconitase-encoding OsACO1 gene alter iron homeostasis. Plant Mol Biol 104, 629–645 (2020). https://doi.org/10.1007/s11103-020-01065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-020-01065-0