Abstract
Various aryl nitriles were readily synthesized from aerobic oxidation of substituted benzyl alcohols in deep eutectic solvent composed of choline chloride (ChCl) and p-toluenesulfonic acid (p-TsOH) in the presence of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), followed by condensation with hydroxylamine hydrochloride. High yields of corresponding nitriles have been obtained under mild reactions conditions. This strategy belongs to a novel and environmentally benign transition-metal-free one-pot cascade process for the synthesis of nitriles.
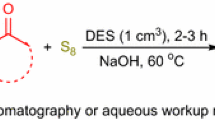
Graphic abstract
Various aryl nitriles were readily synthesized from aerobic oxidation of substituted benzyl alcohols in deep eutectic solvent (ChCl/p-TsOH) in the presence of TEMPO, followed by condensation with hydroxylamine hydrochloride. This strategy belongs to a novel and environmentally benign transition-metal-free one-pot cascade process for the synthesis of nitriles.



Similar content being viewed by others
References
Ragab F A, Gawad N M A, Georgey H H and Said M F 2013 Synthesis of novel 1,3,4-trisubstituted pyrazoles as anti-inflammatory and analgesic agents Eur. J. Med. Chem. 63 645
Fleming F F, Yao L, Ravikumar P C, Funk L and Shook B C 2010 Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore J. Med. Chem. 53 7902
Berteotti A, Vacondio F, Lodola A, Bassi M, Silva C, Mor M and Cavalli A 2014 Predicting the reactivity of nitrile-carrying compounds with cysteine: a combined computational and experimental study ACS Med. Chem. Lett. 5 501
Yamaguchi K, Matsushita M and Mizuno N 2004 Efficient hydration of nitriles to amides in water, catalyzed by ruthenium hydroxide supported on alumina Angew. Chem. Int. Ed. 43 1576
Benz P, Muntwyler R and Wohlgemuth R 2007 Chemoenzymatic synthesis of chiral carboxylic acids via nitriles J. Chem. Technol. Biotechnol. 82 1087
Zhou C and Larock R C 2004 Synthesis of aryl ketones by the Pd-catalyzed C-H activation of arenes and intermolecular carbopalladation of nitriles J. Am. Chem. Soc. 126 2302
Jnaneshwara G K, Deshpande V H, Lalithambika M, Ravindranathan T and Bedekar A V 1998 Natural kaolinitic clay catalyzed conversion of nitriles to 2-oxazolines Tetrahedron Lett. 39 459
Sandmeyer T 1884 Ueber die Ersetzung der Amid-gruppe durch Chlor, Brom und Cyan in den aromatischen Substanzen Ber. Dtsch. Chem. Ges. 17 2650
Enthaler S 2011 Straightforward uranium-catalyzed dehydration of primary amides to nitriles Chem. - Eur. J. 17 9316
Kim H S, Kim S H and Kim J N 2009 Highly efficient Pd-catalyzed synthesis of nitriles from aldoximes Tetrahedron Lett. 50 1717
Fang C, Li M, Hu X, Mo W, Hu B, Sun N and Shen Z 2016 A mild TEMPO-catalyzed aerobic oxidative conversion of aldehydes into nitriles Adv. Synth. Catal. 358 1157
Zhang Y, Xu K, Chen X, Hu T, Yu Y, Zhang J and Huang J 2010 Highly selective aerobic oxidation of primary amines to nitriles by ruthenium hydroxide Catal. Commun. 11 951
Zhang Y, Zhao X, Zhang H, Yan X and Zhao J 2016 Conversion of benzyl alcohol to benzonitrile over a Cu10.3/SiO2 catalyst Appl. Catal. A 522 45
Zhang Y, Zhang Y, Feng C, Qiu C, Wen Y and Zhao J 2009 Amination of ethanol to acetonitrile over Ni-doped Co/γ-Al2O3 catalyst Catal. Commun. 10 1454
Oishi T, Yamaguchi K and Mizuno N 2009 Catalytic oxidative synthesis of nitriles directly from primary alcohols and ammonia Angew. Chem. Int. Ed. 48 6286
Hu Y L, Wang B T and Fang D 2017 Facile and efficient preparation of nitriles through FeCl4-IL-SiO2-catalyzed direct oxidation of alcohols with hydrogen peroxide J. Iran. Chem. Soc. 14 233
Molla R A, Ghosh K, Tuhina K and Islam S M 2015 An aerobic oxidative synthesis of aryl nitriles and primary aryl amides from benzylic alcohols catalyzed by a polymer supported Cu(II) complex New J. Chem. 39 921
Reddy K R, Maheswari C U, Venkateshwar M, Prashanthi S and Kantam M L 2009 Catalytic oxidative conversion of alcohols, aldehydes and amines into nitriles using KI/I2–TBHP system Tetrahedron Lett. 50 2050
Kazemnejadi M, Nikookar M, Mohammadi M, Shakeri A and Esmaeilpour M 2018 Melamine-Schiff base/manganese complex with denritic structure: an efficient catalyst for oxidation of alcohols and one-pot synthesis of nitriles J. Colloid Interface Sci. 527 298
Preger Y, Root T W and Stahl S S 2018 Platinum-based heterogeneous catalysts for nitrile synthesis via aerobic oxidative coupling of alcohols and ammonia ACS Omega 3 6091
Ishida T, Watanabe H, Takei T, Hamasaki A, Tokunaga M and Haruta M 2012 Metal oxide-catalyzed ammoxidation of alcohols to nitriles and promotion effect of gold nanoparticles for one-pot amide synthesis Appl. Catal. A 425 85
Azarifar D and Najminejad Z 2015 Direct oxidative conversion of benzylhalides, -amines, -alcohols, and arylaldehydes to nitriles with trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane activated by NH4Br J. Iran. Chem. Soc. 12 107
Iida S and Togo H 2007 Direct oxidative conversion of alcohols and amines to nitriles with molecular iodine and DIH in aq NH3 Tetrahedron 63 8274
Dighe S U, Chowdhury D and Batra S 2014 Iron Nitrate/TEMPO: a superior homogeneous catalyst for oxidation of primary alcohols to nitriles in air Adv. Synth. Catal. 356 3892
Dornan L M, Cao Q, Flanagan J C, Crawford J J, Cook M J and Muldoon M J 2013 Copper/TEMPO catalysed synthesis of nitriles from aldehydes or alcohols using aqueous ammonia and with air as the oxidant Chem. Commun. 49 6030
Tao C, Liu F, Zhu Y, Liu W and Cao Z 2013 Copper-catalyzed aerobic oxidative synthesis of aryl nitriles from benzylic alcohols and aqueous ammonia Org. Biomol. Chem. 11 3349
Zhang Y, Huang R, Gao B and Zhao J 2016 Solvent-free aerobic oxidation of alcohols to nitriles catalyzed by copper iodide in combination with a quaternary ammonium modified TEMPO Catal. Lett. 146 220
Chinnusamy T 2019 Recyclable MeOPEG-clicked TEMPO catalyst for one-pot aerobic double dehydrogenation of alcohols to nitriles Catal. Commun. 119 51
Jiang Y, Sun B, Fang W Y and Qin H L 2019 A transition-metal-free one-pot cascade process for transformation of primary alcohols (RCH2OH) to nitriles (RCN) mediated by SO2F2 Eur. J. Org. Chem. 2019 3190
Li Z, Wang T, Qi X, Yang Q, Gao L, Zhang D and Wang Y 2019 Green synthesis of benzonitrile using ionic liquid with multiple roles as the recycling agent RSC Adv. 9 17631
Xu Y, Jia X, Ma J, Gao J, Xia F, Li X and Xu J 2018 Efficient synthesis of 2,5-Dicyanofuran from biomass-derived 2,5-Diformylfuran via an oximation-dehydration strategy ACS Sustain. Chem. Eng. 6 2888
Nakajima M, Qiao K, Kobayashi N, Bao Q, Tomida D and Yokoyama C 2011 Efficient dehydration of aldoximes to nitriles catalyzed by a Lewis acid ionic liquid Chem. Lett. 40 396
Miao C X, He L N, Wang J Q and Wang J L 2009 TEMPO and Carboxylic Acid Functionalized Imidazolium Salts/Sodium Nitrite: An Efficient, Reusable, Transition Metal-Free Catalytic System for Aerobic Oxidation of Alcohols Adv. Synth. Catal. 351 2209
Liu R, Liang X, Dong C and Hu X 2004 Transition-metal-free: A highly efficient catalytic aerobic alcohol oxidation process J. Am. Chem. Soc. 126 4112
Karimi B, Biglari A, Clark J H and Budarin V 2007 Green, transition-metal-free aerobic oxidation of alcohols using a highly durable supported organocatalyst Angew. Chem. Int. Ed. 46 7210
Hirashita T, Nakanishi M, Uchida T, Yamamoto M, Araki S, Arends I W and Sheldon R A 2016 Ionic TEMPO in ionic liquids: Specific promotion of the aerobic oxidation of alcohols ChemCatChem. 8 2704
Liu R, Dong C, Liang X, Wang X and Hu X 2005 Highly efficient catalytic aerobic oxidations of benzylic alcohols in water J. Org. Chem. 70 729
Miller S A, Bisset K A, Leadbeater N E and Eddy N A 2019 Catalytic oxidation of alcohols using a 2,2,6,6-tetramethylpiperidine-N-hydroxyammonium cation Eur. J. Org. Chem. 2019 1413
Wang X, Liu R, Jin Y and Liang X 2008 TEMPO/HCl/NaNO2 Catalyst: A transition-metal-free approach to efficient aerobic oxidation of alcohols to aldehydes and ketones under mild conditions Chem. - Eur. J. 14 2679
Abbott A P, Boothby D, Capper G, Davies D L and Rasheed R K 2004 Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids J. Am. Chem. Soc. 126 9142
Dai Y, van Spronsen J, Witkamp G J, Verpoorte R and Choi Y H 2013 Natural deep eutectic solvents as new potential media for green technology Anal. Chim. Acta 766 61
Paiva A, Craveiro R, Aroso I, Martins M, Reis R L and Duarte A R C 2014 Natural deep eutectic solvents–solvents for the 21st century ACS Sustain. Chem. Eng. 2 1063
Wen Q, Chen J X, Tang Y L, Wang J and Yang Z 2015 Assessing the toxicity and biodegradability of deep eutectic solvents Chemosphere 132 63
Zhang Q, Vigier K D O, Royer S and Jérôme F 2012 Deep eutectic solvents: syntheses, properties and applications Chem. Soc. Rev. 41 7108
Smith E L, Abbott A P and Ryder K S 2014 Deep eutectic solvents (DESs) and their applications Chem. Rev. 114 11060
Yuan L, Yin G, Zhang H Y, Zhang Y and Zhao J 2018 Aerobic oxidative conversion of benzylic alcohols with ammonia to nitriles catalyzed by CuCl/TEMPO/PIC Chem. Pap. 72 2679
Iwabuchi Y 2013 Discovery and exploitation of AZADO: the highly active catalyst for alcohol oxidation Chem. Pharm. Bull. 61 1197
Cao Q, Dornan L M, Rogan L, Hughes N L and Muldoon M J 2014 Aerobic oxidation catalysis with stable radicals Chem. Commun. 50 4524
Ciriminna R and Pagliaro M 2010 Industrial oxidations with organocatalyst TEMPO and its derivatives Org. Process Res. Dev. 14 245
Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (Grant No. 21776056).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information (SI)
Supplementary Information (SI)
Supplementary information related to this article is available at www.ias.ac.in/chemsci.
Rights and permissions
About this article
Cite this article
Liu, L., Zhang, HY., Yin, G. et al. Deep eutectic solvent promoted one-pot synthesis of nitriles from alcohols. J Chem Sci 132, 122 (2020). https://doi.org/10.1007/s12039-020-01815-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01815-z